Pump thrombosis—A riddle wrapped in a mystery inside an enigma
Introduction
With all due apologies to Winston Churchill, the subheading of this keynote lecture signals how little is known about the subject of pump thrombosis (PT): it is a difficult problem to define, it is a difficult problem to understand, it is a difficult problem to diagnose, and it is a difficult problem to prevent and treat. Although guidelines have been recently published of how to best diagnose and manage patients presenting with ventricular assist device (VAD) thrombosis, most—if not all—of the guidelines are based on expert or consensus opinion and not on randomized trials (1). Hence, there is still room for substantial improvement and mastery in knowledge as we continue to try to tackle this thwarting issue.
Overall, the field of mechanical circulatory support (MCS) has been experiencing several salutary trends over recent years. First, the U.S. population is aging. Over the next 20 years, there will be a doubling of the population over 65 (2). Importantly, because the incidence of heart failure (HF) rises with patient age, this portends a substantial growth in the HF demographic. Since most patients with advanced HF will not be candidates for heart transplantation by virtue of age alone, this suggests a substantial increase in the potential demand for MCS—other things being equal.
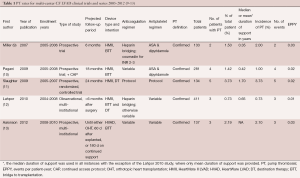
Second, the survival of patients with continuous flow (CF) left VADs (LVADs) continues to improve, with approximately 80% one-year survival according to the data from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) database (Figure 1) (3). Generally, survival declines by approximately ten percentage points each year thereafter—80% at one year, 70% at two years, etc. Moreover, quality of life measures indicate a marked improvement after LVAD implantation. Hence, notwithstanding the severe morbidities experienced by LVAD patients, survival and overall quality of life is vastly enhanced with MCS (Figure 1) (3).
Third, in recent years, and particularly in the U.S., there has been an expansive growth in centers getting certified for destination therapy (DT). With the increasing number of certified centers, patient access to MCS will expand. In addition, there has been a shift away from patients undergoing LVAD implantation for bridge to transplantation (BTT) towards DT. At present, patient enrollment in INTERMACS has increased by approximately 2,500 patients per year, and over 40% of these patients are undergoing LVAD placement for DT in the most recent era (3). Significantly, this patient accrual underestimates the true growth in volume, given that the INTERMACS database is restricted to only those patients receiving devices under FDA-approved indications.
Fourth, as the data continues to accumulate, it is clear that there is a clear and significant survival advantage for patients graded as INTERMACS 3-7 as compared to those in INTERMACS 1 or 2 (3,4). This has impelled many in the field to call for further studies examining the benefit of LVAD therapy in patients with even lesser degrees of HF. Two such studies enrolling patients with lesser degrees of HF include ROADMAP and REVIVE-IT. ROADMAP is an observational study comparing outcomes of LVADs in patients in INTERMACS 4-6, within the current labeling of HeartMate II LVAD (HMII), and comparing them to outcomes with optimal medical management. REVIVE-IT is a randomized prospective trial enrolling patients in NYHA class III, most of whom will be outside of the current labeling for HeartMate II (5).
Hence, the landscape is such that the growth of MCS is favored by a number of market drivers: the target population is growing, more centers are implanting, more patients are undergoing implantation, patient selection and outcomes are improving, and the target population may soon be expanding if the ROADMAP and REVIVE-IT trials are successful.
Review of the published clinical data published before 2013
Despite the survival and quality-of-life benefits of MCS, the field has been plagued by complications that will need to be better tackled in coming years if the therapy is to thrive. These complications include right ventricular failure, infection, aortic insufficiency (AI), hemorrhage, stroke, and LVAD thrombosis. In particular, LVAD PT is a daunting problem because it has been increasing in incidence in recent years—without clear explanation—and is associated with a worse prognosis (6-8). Moreover, as will be elaborated upon shortly, the other complications of MCS and their prevention and management appear to be contributing to the growing PT problem.
Prior to 2013, the literature on PT had been relatively inconspicuous (vide infra). The data from clinical trials and series, and occasional case reports, had suggested a relatively rare occurrence of PT in comparison to other complications. Publications from this period of time all reflected PT rates less than 0.03 events per patient year (EPPY), which compared quite favorably to the rates of the other major morbidities. Epidemiologically, PT—though irksome—thus appeared to be an uncommon phenomenon.
Table 1 lists the PT data for multicenter studies including more than 100 patients and in which PT events were able to be either extracted or calculated from the data (9-13). Three remarks are made here with respect to the PT thrombosis literature.
First, the convention we will use in this manuscript is to focus on the EPPY rates, since this calculation will minimize—but not eliminate—disparities in support duration that might obfuscate the incidence rates. Regardless, the EPPY rate may not give a very comparable reflection across studies, since in those studies where the time to PT is long relative to mean or median duration of support, some patients might not have developed evidence of PT yet.
Second, one has to carefully define what counts as a PT event for each study. PT can be defined strictly as confirmed PT, wherein a pathological diagnosis is made at the time of LVAD exchange, explant, heart transplantation, or death. PT can also be more loosely defined as including suspected PT in addition to confirmed cases. Suspected cases are those where a diagnosis is based on clinical data but no pathological diagnosis is obtained. Hence, studies that include both confirmed and suspected PT cases will overstate the incidence of PT in comparison to those including confirmed PT alone. All the data in Table 1 is restricted to confirmed PT cases, and it will be the convention in this manuscript to point out which category of PT is applicable when discussing each study.
Third, the anticoagulation (AC) and antiplatelet regimens often varied from study to study, from center to center within studies, and even within single centers during the course of a study. Hence, there is quite a bit of variability in the regimens across both time and space. Firm conclusions regarding the merits of specific AC and antiplatelet regimens based on these studies are therefore fraught with hazard.
Most of the relevant data regarding PT rates prior to 2013 arise from patients enrolled in the landmark prospective studies along with their continued access protocols (CAP) (9-13). Because of the HeartMate II’s market presence longer than that for the HeartWare LVAD (HVAD), most of the data that is available is for the former.
HeartMate II (HMII) studies
Two publications reported the results from the original HMII BTT trial and updated results including the patients in its CAP (9,10). Miller et al. published the results of the HMII BTT trial in 2007, which enrolled a total of 133 patients during 2005-2006. In this trial, most patients were bridged with heparin, and warfarin was administered for a target INR of 2.0-3.0. Aspirin (ASA) and dipyridamole were also used routinely. The PT rate was calculated based on the number of surgical explants for PT, so these were all confirmed PTs. At a median duration of support of 126 days, 2 of the 133 patients (1.5%) developed PT. The EPPY rate was 0.03 (Table 1) (9).
In the 18-month analysis of the HMII BTT trial including patients in the CAP, 281 patients were enrolled. PT was defined, as per the earlier BTT trial, as including only confirmed cases. At a median support duration of 155 days, four of the 281 patients developed PT, with an incidence rate of 1.42%. Three of the four patients developed PT within two months of implantation. The EPPY rate was 0.02 (10). Hence, for both the original BTT trial along with later results including the CAP cohort, EPPY rates were similar (Table 1).
The HMII DT trial, which was a randomized prospective study comparing the HMII against the HM XVE, was published by Slaughter and colleagues in 2009. The AC regimen was variable during the course of the trial due to the high bleeding rates encountered in the BTT trial (vide infra). PT was diagnosed after explantation, with a level 3 thrombus (>50% obstruction) counting as an event. At a median duration of support of 620 days, five of the 142 (3.7%) HMII patients developed PT. The EPPY was 0.02, mirroring those from the BTT studies (Table 1) (11).
The combined European HMII BTT and DT experience from 2004 to 2008 was reported upon by Lahpor et al. (12). This was a multi-institutional study in which patients were followed for at least six months after surgery, however there was no mention of how PT was diagnosed. Of the 411 patients, three (0.73%) developed PT, with a mean support duration of 236 days. Although this study only included the mean and not median durations of support, the EPPY was 0.01—very low and not likely to be much different if the median duration of support was used in the calculation (12) (Table 1).
Hence, the clinical trial data seemed to indicate an overall PT rate for the HMII of a tolerable 0.01 to 0.03 EPPY in publications from prior to 2013 (Table 1).
HVAD studies
Aaronson et al. published the results for the HVAD BTT study that included 137 patients enrolled during 2008-2010. Patients were followed until either heart transplantation, 60 days following explantation in recovered patients, or 180 days for patients on continued support. The AC protocol was variable. All PTs were confirmed at explantation. Although the median duration of support was not mentioned in the report, three of 137 patients (2.2%) were diagnosed with PT, with an EPPY rate of 0.03 (Table 1) (13).
Evolution of AC and antiplatelet management
In the original HMII BTT trial, the protocol recommendations were to bridge patients with heparin postoperatively, to anticoagulate the patients with warfarin to an INR of 2.0-3.0, and to place patients on low dose ASA (14,15). With that strategy, the perioperative and long-term bleeding rate was high, and notably, bleeding was the most frequent morbidity observed during the HMII BTT trial. Over 50% of patients had bleeding that required the transfusion of more than two units of packed red cells and over 30% of patients had bleeding that required surgery (9). Given the low reported PT rates in earlier experiences described above, numerous centers began experimenting with alternative strategies, such as bypassing heparin bridging and shooting for a lower INR target.
The aforementioned trends in AC management were explored in two retrospective studies appearing in the literature in 2009 and 2010. Boyle et al. analyzed the outcomes of patients maintained with a lower INR (15), and Slaughter et al. examined the outcomes with foregoing heparin bridging (14).
In the Boyle study, the authors reviewed the data on patients who were enrolled in the HMII pivotal BTT trial and who were at least a month out from hospital discharge (15). The 331 patients included were retrospectively divided into five INR groupings, and bleeding and thromboembolic events were analyzed for each of these groupings. The two important findings from the study were as follows: first, all patients with an INR >1.5 had low PT rates, and there was no apparent advantage of having a higher INR. Second, bleeding events were increased in patients with an INR above 2.5. Hence, Boyle’s group concluded that the optimal target range for the INR should be between 1.5 and 2.5, instead of the 2.0 to 3.0 in the original HMII protocol, effectively downshifting the INR target by 0.5. Thus, the results suggested that a lower INR target was safe from the perspective of PT.
Next, Slaughter and colleagues focused on the necessity for heparin bridging in patients undergoing HMII implantation in 35 centers (14). A total of 418 patients from the BTT trial who had been on LVAD support for at least seven days were included. The patients were retrospectively assigned to one of three groups based on postoperative PTT values:
- Group A (therapeutic, n=118) patients received intravenous heparin and had two or more PTT values >55 seconds;
- Group B (sub-therapeutic, n=178) patients received intravenous heparin at a low dose or intermittently and had at least one PTT value >40 seconds, but did not have more than one value >55 seconds;
- Group C (no heparin, n=122) patients did not receive heparin therapy and none had a PTT value >40 seconds.
The study found that patients in group C, who were not bridged with heparin, were not at any greater risk for thromboembolic complications and had significantly less bleeding in the 30 days after surgery in comparison to the other groups. Hence, the authors concluded, bridging with heparin may not be necessary in patients who are at low thromboembolic risk, and may be safer from the standpoint of bleeding.
These two published studies thus served to embolden the emerging practices of aiming for a lower INR target and abandoning heparin bridging for most patients (16).
PT revisited: 2013 and beyond
Soon Park and colleagues in 2012 published a comparison of the outcomes of the initial HMII DT trial cohort with the patients in the CAP (17). The initial cohort was enrolled from March 2005 through May 5, 2007; the CAP cohort was enrolled from May 5, 2007 through March 31, 2009. Patients were followed for at least two years following implant, and the diagnosis of PT was confirmed at device explantation (i.e., same criteria as the original trial). In their analysis, they found a relative risk of PT and pump replacement for PT to be 1.6 and 1.7, respectively, in the latter cohort; however, this trend was not statistically significant (Table 2).
Moreover, Park and colleagues analyzed the AC regimen of the two groups and discovered that a higher percentage of patients in the CAP group were not bridged with heparin (38% vs. 22%, P<0.004), and the average INR was lower in the CAP group (1.8 vs. 1.9, P=0.028) as compared to the original BTT cohort. These AC trends are expected given the previously discussed changes in the attitudes regarding AC, but at best they imply an association between less intense AC and a trend towards an increasing incidence of PT.
In January 2014, several publications attracted national and worldwide attention for those in the MCS field. Three studies were published in two journals in that single month that retrospectively reviewed more recent data on PT (6-8). The first described the relationship between the now BTT-approved HVAD and VAD thrombosis, and the other two looked at the relationship between the HMII and VAD thrombosis. Each of these studies will be discussed in turn. A more exhaustive treatment of the comparative analysis among these studies is provided by Mehra et al., and this analysis appeared in the same issue of the Journal of Heart and Lung Transplantation as two of the aforementioned studies (16).
Najjar and colleagues described the evolving experience with PT with the HVAD (7). The study population included 382 patients who were either part of the original BTT trial cohort [2008-2010] or were included in the CAP group [2010-2012]. PT was defined as an event occurring >72 hours after implantation that included alterations in pump parameters, an associated increase in biochemical markers of hemolysis, visualization of organized fibrin in the pump on explantation, and/or abnormal pump sounds by auscultation. Hence, the definition was fairly broad and included cases of both confirmed and suspected PT. The following were the major findings:
- A total of 34 PT events occurred in 31 patients (8.1% of the cohort, 0.08 EPPY);
- The incidence of PT did not differ when comparing the BTT and CAP groups.
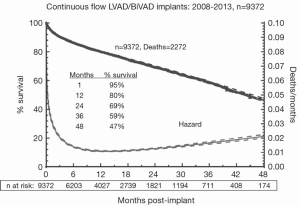
In the original design of the HVAD, both internal and external surfaces of the inflow cannula were made almost exclusively of highly polished titanium alloy, as seen in the upper left panel of Figure 2. At explantation of the hearts during heart transplantation, examination of these hearts revealed tissue ingrowth encroaching upon the open inflow end of the cannula, as seen in the bottom left panel. This finding inspired the conversion to a partially sintered titanium inflow cannula, as seen in the upper right panel. The typical pathological appearance of the latter cannula is seen in the lower right panel. It is as if the sintered titanium creates a moat that the tissue ingrowth cannot travel across.
Interestingly, the thrombosis rates for the two configurations were not significantly different in this report; however, the median follow-up time was much shorter for the sintered titanium group (309 days) in the context of a fairly long median time to PT (245 days). Hence, it is likely that some patients who would have experienced PT were missed, given the relatively short follow-up time.
On multivariate analysis, four risk factors for PT figured prominently and are as follows:
- Being on ASA doses at or below 81 mg/d;
- Having an INR <2;
- Having a less ill patient profile of INTERMACS 3-7;
- Having a MAP >90.
The presence of any one of these factors more than doubled the risk of a PT event. The first two listed factors are easy to explain in light of the studies mentioned earlier, however, the less sick patient profile and the higher MAP are not intuitively obvious risk factors. The high MAP association is definitely interesting, and one wonders whether the higher MAPS were associated with lower VAD flows, as would be expected. If indeed the flows were lower in these patients, one could hypothesize that the increased PT rate in these patients may be due to less heat dissipation in the pump. This risk factor is worthy of further examination. The propensity for the less ill INTERMACS levels to have PT is unclear, and may perhaps be explained by other unrecognized factors contributing to this phenomenon in this group of patients. The authors allude to the fact that more patients in the less ill group were sent home without antiplatelet therapy, but this should have been rectified in the multivariate analysis.
Regardless, these findings prompted significant changes in the postoperative protocol for HVAD patients. Most centers now have increased the ASA to 162 mg per day, increased the INR target to 2.0-3.0, and maintain the mean arterial pressure below 90 mmHg.
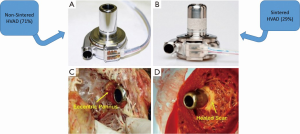
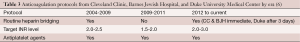
In the same month, Starling and colleagues from three high-volume U.S. VAD centers published data harvested over a nine-year period regarding the incidence of PT (6). These institutions included the Cleveland Clinic, Barnes Jewish Hospital, and Duke University Medical Center. Two categories of PT were recognized: confirmed PT included patients who had direct pathological evidence of thrombus at pump replacement, heart transplantation, or autopsy; suspected PT included those patients who had pump malfunction suggestive of PT, but who had no pathological confirmation.
Figure 3 shows the contribution of patients from each institution, and the overall occurrence of confirmed PT at three months after HMII implantation. Importantly, the graph illustrates that there was an abrupt increase in the incidence of PT at each of the institutions with an inflection point occurring in March 2011. At three months after implantation, the confirmed PT rate rose almost fourfold from 2.2% prior to March 2011 to 8.4% by 2013. In addition, the median time to confirmed PT went from 18.6 months prior to March 2011 to 2.7 months after March 2011, a reduction by greater than 80%.
Table 3 summarizes the AC protocol of the three institutions. There are several points worthy of note: heparin bridging was generally used prior to 2009 and resumed after 2011, without heparin bridging in the intervening period. CCF and BJH begin it immediately after implant if there is minimal bleeding, and Duke begins if the INR is not therapeutic in three days. INR targets fluctuated over the eras from a higher target, down to a lower target, and back up to a higher target in the most recent era. All programs used antiplatelet therapy with ASA throughout the eras.
Full table
Prompted by concerns from groups regarding a perceived increase in PT rates, Kirklin and colleagues reported data derived from the INTERMACS database (8). All episodes of PT were confirmed at device exchange or autopsy. Figure 4 shows Kaplan Meier event curves by implant year showing the freedom of device exchange or death due to PT. The INTERMACS database thus confirms the findings of Starling’s group: over the period from 2008 to 2012, the six month PT rate went up six-fold, from 1% to 6%. Furthermore, those undergoing pump exchanges for thrombosis had a worse overall survival as compared to patients undergoing their primary VAD.
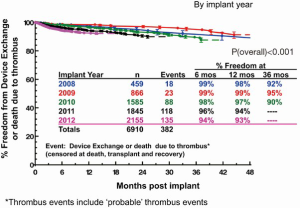
In the INTERMACS study, the following were found to be risk factors for device exchange or death due to PT: implant year, younger age, white race, larger BMI, LVEF >20%, and higher LDH at one month. Some of these are intuitive, others require a bit more thought and exploration.
After the January 2014 “triple onslaught” of articles on PT, several other publications have appeared. Columbia University published their experience with HMII PT from 2009-2012. Nineteen of 177 patients (11%) developed PT after a mean of 351±311 days, for an EPPY rate of 0.12. Postoperatively, most patients were not bridged with heparin unless extubation was delayed by more than 72 hours. The target INR in recent years has been 1.5-2.5, all patients are on ASA 81 mg per day, and patients younger than 50 years are also placed on dipyridamole. A root cause analysis of PT was performed by this group, and the details appear in a later section of this manuscript (18).
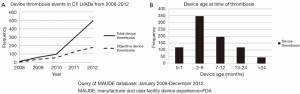
One final study to add briefly, presented at the 2014 ISHLT meeting, includes data from the MAUDE database (19). This database collects post-market surveillance data on FDA-approved medical devices. As seen in Figure 5, the Maude database confirms the increase in PT, but the inflection point here starts about a year earlier than for the Starling study, in 2010. Corroborating the results from Starling’s paper, the peak incidence of device thrombosis in the MAUDE database occurred between two and six months post implant. It should be remembered that some cross population of studies by patients in the Starling, Kirklin, and MAUDE articles exists.
In summary, as one might imagine, the issue of mounting VAD thrombosis rates has had quite a chilling effect on those in the field. It seems as if, just as we have approached the holy grail of hoping to extend MCS to the higher and less ill INTERMACS levels, the field is immersed in ice cold water.
For, how can one expect to extend MCS to earlier stage HF patients in this milieu?
Etiology of PT and its recent rise in incidence
In this section we will describe the types of thrombi, their origins, a framework for causative factors, and a rationale for the recent uptick in PT incidence.
Types of thrombi
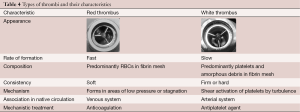
There are two basic types of PT, including red thrombus and white thrombus (20,21). Table 4 lists the features that contrast the two types. Red thrombi are usually responsible for acute catastrophic pump thromboses, whereas white thrombi are responsible for the more insidious PT that present subacutely (21). The composition of red thrombi consists predominantly of red blood cells trapped in a fibrin mesh; the composition of white thrombi consist predominantly of aggregated platelets along with debris. Red thrombus is soft; white thrombus is firm or hard. The mechanism of formation for red thrombus is coagulation of stagnant blood; that for white thrombus is shear activation of platelets. The implied treatment for these thrombi is based on their mechanisms: thrombolytics for red thrombi, and glycoprotein IIb/IIIa inhibitors for white thrombi (20,21).
Full table
In a recent ISHLT abstract, 10 of 130 (7.7%) patients developed PT requiring either pump replacement or removal. Nine were composed of red clots; one was composed of white clot (21).
Complicating matters further, of patients that go to the operating room for pump exchange, many have elements of both red and white thrombus.
Origins of PT
What ultimately becomes a VAD thrombus can be initiated in the VAD de novo or could arise elsewhere and either extend or embolize to the VAD. In the absence of an obvious mechanical factor, it is rare to know the exact origin of the thrombus or embolus. Generally speaking, the thrombus may have formed in the blood upstream to the VAD, it may have formed de novo in the pump body itself, or it may propagate proximally to the body by distal obstruction or turbulence in the outflow graft (Figure 6).
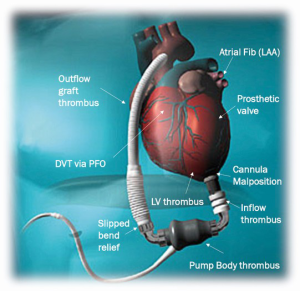
Frameworks for understanding PT
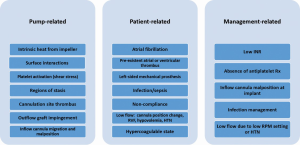
Goldstein and colleagues published a “Perspective on VAD thrombosis” that contributed two important frameworks for the diagnosis and management of patients with PT. The first framework, as depicted in Figure 7, categorizes the etiologic factors for PT as either pump-related, patient-related, or management-related. It is a very useful scheme for understanding the interplay of factors in a given patient, even though there may be some degree of overlap across the categories. The second framework consists of an algorithm for the diagnosis and management of PT and will be discussed in later sections. Given the paucity of randomized studies in this area, it must be remembered that most of the recommendations are based on expert and consensus opinions. Nonetheless, the framework provides an excellent foundation upon which to lay accumulating evidence as it occurs.
While an exhaustive discussion of the factors contributing to PT is beyond the scope of this publication, a few items derived from the foregoing etiologic framework are of particular significance and are discussed next.
Pump-related factors
Pump-related factors include interactions at the interface between blood and the components of the pump (Figure 7). The impeller mechanism involves interactions which include heat generation, surface interfaces, shear stress, and stasis. For the inflow portion, they include thrombus formation at the cannulation site and cannula malposition with obstruction. For the outflow portion, they include outflow graft impingement by the bend relief, graft kinking or twisting, and obstruction of the outflow anastomosis (1).
Numerical simulations, which have been instrumental in optimizing VAD designs, have also been assuming an increasing role in the assessment of PT. Constructing the VAD simulations is a formidable challenge, since blood biochemistry, mechanobiology, and physiological response are often very difficult to model (22). The calculations rely on solving specialized equations of incompressible viscous flow, representing the properties of blood. The simulations are generated by algebraic equations governing the conservation laws for mass, momentum, and energy. Adding to the complexity of these calculations is that CF VADs in particular present special challenges that require tackling by tools designed to analyze flows involving both rotating and stationary components. Although the complexities of numerical simulations are beyond the scope of this manuscript, the reader is referred to an outstanding exposition on the use of numerical simulations in VAD design and analysis (22).
Chiu et al. (23) used numerical simulations to predict areas prone to thrombus formation in the HMII VAD. The group discovered stagnant platelet trajectories just distal to the flow straightener and at the front edge of the actual impeller. In addition, recirculation zones were observed spanning the area just downstream of the flow-straightener. These findings correlate quite closely with what we observe clinically.
The model of pump may have a bearing on the propensity for PT, but as of yet, we only incompletely understand the hematologic profiles of each pump. The HMII is an axial flow pump, and the HVAD is a centrifugal flow pump, and one would expect that the interactions with circulating blood would be different. In a recent study, Birschmann and colleagues found that the HVAD is associated with lower LDH levels than is the HMII, but that the HMII is associated with lower levels of fibrin split products (24). The implications of these differences in hemolysis and fibrinolysis for the relative incidences of PT in the two pumps are unclear at present.
Earlier renditions of both the HMII and the HVAD have been modified to make them less thrombogenic through the inclusion of sintered titanium surfaces in select areas (7). Sintered titanium promotes the formation of a densely adherent pseudo-intima, which hinders the direct interface between the prosthetic material and blood elements—thereby impeding thrombus formation (6). In Najjar’s series, pathological specimens showed a remarkable distinction between the heart specimens obtained from patients with the non-sintered versus the sintered titanium inflow cannulae (7). As previously described, while there was impressive tissue ingrowth towards the inflow aperture in the former, there was almost none beyond the sintered portion in the latter (Figure 2). It is likely that most future formulations of CF LVADs will include sintering or some variant thereof.
Patient-related factors
Patient-related factors include factors that are present prior to LVAD placement as well as those that develop afterwards (Figure 7). Pre-existing factors include atrial fibrillation, left atrial or left ventricular thrombus, left-sided mechanical prosthesis, and hypercoagulable states. Pre-existent atrial fibrillation nearly doubles the risk of a thromboembolic event after LVAD placement (25). Some of the foregoing factors, if not present at the time of VAD placement, may occur later.
Of particular concern is the emergence in some reports of HIT syndrome as an etiologic factor in some cases of PT (26-28). Of the four patients who underwent pump exchange for PT at one European center, three were diagnosed with HIT. Because its diagnosis may be variably pursued in some centers, it is more than likely that the incidence of HIT-related PT is underestimated. Some in the field have argued that, because of the relatively high incidence of HIT antibody in LVAD patients and the difficulty of delineating which patients have true HIT syndrome, it may be advisable to replace heparin perioperatively with non-heparin intravenous anticoagulant agents such as argatroban (27).
New patient-related issues that may occur at any time after VAD implantation include infection or sepsis, evidence of noncompliance, and low-flow states. Special diligence is warranted in patients who develop infections after VAD placement (vide infra).
Whereas noncompliance is a factor precluding heart transplantation in some patients, these patients—particularly the young-may at times be considered for DT LVAD placement with the possibility of transitioning to transplant candidacy if compliance is demonstrated in the future. Unfortunately, many of these patients continue their old recalcitrant ways and may display a disregard for their medications, which places them at substantial risk of developing PT.
Low-flow states may include those that lead to lower preload of the LVAD or greater afterload. A lowered preload can be seen with cannula position migration, right-sided HF, and hypovolemia. A publication by Taghavi et al. examined whether there may be factors evident on a routine chest X-ray that might be predictive of PT (29). The group found that HMII patients who developed PT in their series had greater acute angulation of the inflow cannula and a decreased pump pocket depth. Both of these characteristics may be surrogates of cannula malposition or migration. It is likely that this phenomenon would not occur in the HVAD patients, since the HVAD is completely intrapericardial and does not require a pump pocket. While cannula malposition may lead to low LVAD flow in the setting of an adequately filled left ventricle, both right-sided failure and hypovolemia lead to a lower preload in the left ventricle itself and therefore a lower preload relayed to the LVAD. Low-flow states may lead to a greater tendency for PT because the decreased flow results in decreased washout of heat generated by the pump, also keeping in mind that heat is a known platelet activator.
Increased afterload may be seen in patients who are hypertensive, and the higher afterload will decrease pump flow for any given revolutions per minute (rpm) setting. In the HVAD BTT study, hypertension was found to be a significant risk factor for PT and prompted a more aggressive stance towards hypertension in LVAD patients (7).
Management-related factors
Management-related factors include a sub-therapeutic INR, absence of antiplatelet therapy, inflow cannula malposition at implant, infection management, intentional efforts to keep pump speed low, and inadequate control of hypertension (Figure 7).
Although prior studies appeared to demonstrate the safety of a lower INR range as well as of bypassing the heparin bridge, more recent data suggests that this may predispose patients towards PT. A subtherapeutic INR and absence of antiplatelet therapy were associated with an increased incidence of PT in the HVAD BTT cohort (7). It is an extraordinary challenge to discover and demonstrate the exact relationship between AC status and PT, given that INR testing is only intermittently performed and the results may widely fluctuate over time. Hence, it is near impossible to have a great degree of control over the intended target range. Antiplatelet status can similarly be a challenge, given the lack of widespread adoption of platelet function testing and the known platelet resistance to either ASA or other antiplatelet agents. Nonetheless, it is evident that patients need to be adequately therapeutic on these agents.
Inadequate recognition and control of infection, or failure to increase AC during infection, may lead to an enhanced thrombosis risk (30,31).
There also appears to be a correlation between patients who had experienced an episode of gastrointestinal (GI) bleeding and subsequent predisposition towards PT, as explored by Stulak et al. (32). In a comparison of LVAD patients with and without GI bleeding, the former had a 7.4 fold increase in thromboembolic events. The hypothesized connection is that an episode of GI bleeding results in a downshifting of AC and antiplatelet management. Presumably, this vulnerable period may sow the seeds for later overt PT. This is an important relationship to recognize, since one of the most frequent serious morbidities after CF LVAD placement is bleeding, as well as one of the most common reasons for readmission (3,9-13).
However, a single center study by Jennings et al. showed that AC reversal was seemingly well tolerated in patients who required reversal (33). One notable difference between the Jennings study and the Stulak study is that, in the latter, thromboembolic events occurring after 30 days were analyzed, whereas in the former events within 30 days were analyzed. There therefore may be a predilection for thromboembolism that manifests itself later in the time course of AC reversal, and therefore the Jennings study may underestimate the thrombogenic risk of stopping AC at the time of bleeding presentation (32,33).
A second potential etiologic mechanism connecting GI bleeding with PT is the trend towards reducing rpms on the CF LVADs in an attempt to open the aortic valve and augment pulsatility—with the hope that this maneuver would decrease the likelihood of arteriovenous (AV) malformation development or progression. Unfortunately, reducing the LVAD rpms in effect leads to lesser washout of heat generation by the pump, and as mentioned previously, heat activates platelets.
Interestingly, while lower pump speeds may predispose toward thrombus formation within the VAD, it may paradoxically decrease thrombus formation within the heart itself. A numerical simulation study pointed to areas of increased stasis in the left ventricle at high pump speeds, and was exacerbated in hearts with the poorest function (34). Clearly, further work and validation needs to be performed in this area.
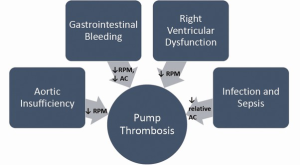
Given the interactions between other MCS complications and PT, Figure 8 diagrammatically summarizes these effects. Either the complication itself, its prevention, or its treatment may lead to PT. As just mentioned, prevention of GI bleeding in VAD patients often includes keeping pump speeds lower to potentially minimize the proliferation of AV malformations. In addition, bleeding, once it occurs, is managed at least temporarily by a halting or decrease in AC. Efforts to decrease the development of AI in LVAD patients include keeping pump speeds on the lower side as well. Right ventricular dysfunction, particularly in the early postoperative period, is likewise managed with lower pump speeds. Finally, infections and sepsis are known to be associated with a more hypercoagulable state in VAD patients. In the end, the occurrence of any of these complications may thus prove to be a surrogate for a higher risk of PT.
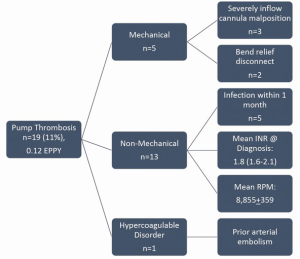
It is clear from the above that the etiology for PT is multifactorial on a general basis and likely for most individual cases. Uriel et al. reported on a root cause analysis performed on all cases of PT at Columbia University Medical Center from 2009-2012 (Figure 9) (18). Nineteen of 177 patients (11%) developed PT after a mean of 351±311 days, for an EPPY rate of 0.12. See Figure 9. Five of the 19 had a mechanical cause either from inflow cannula malposition or bend relief disconnection. One patient had an identifiable hypercoagulability disorder, while the remaining 13 patients had a “non-mechanical” basis for PT. This group had a relatively low mean INR and low pump speed setting, but these values for patients that did not develop PT were not provided for comparison.
Increasing incidence of PT
The above review of the factors contributing to PT now allows for an analysis of the factors that may have contributed to the recently noted increase in PT rates. Mehra et al. have written a perceptive analysis of the rising PT problem, and provide the superlative diagram that is reproduced in Figure 10 (16). It diagrammatically represents the landscape of factors contributing to the uptick in PT rates that have occurred in recent years.
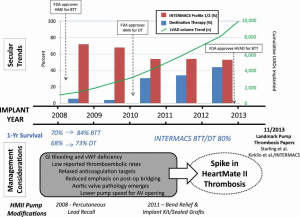
Moving from top to bottom in the figure, the figure tracks the time course of FDA approvals, growth of VAD implantations, shifting of implantations to a less ill population, relative growth in DT implantations, improving survival, changing management considerations, HMII pump modifications, and how these trends may have impacted the occurrence of PT. The paper hones down on the following factors’ being responsible for the spike in PT rates:
- The management of GI bleeding and vWF deficiency that includes decreasing rpm speeds and temporary halting of AC and antiplatelet therapy;
- Low reported thromboembolic rates leading to a relaxed approach to heparin bridging and INR targets;
- Recognition of the late development of AI and attempts to prevent it by lowering pump rpms (16).
It is likely that each of these factors has played an important part in the recent increase in PT rates. Recognition of these contributing factors results in implications for the present and future management of CF LVAD patients that are discussed below. Some of these factors can be modified, others will be more of a therapeutic challenge.
Surveillance and prevention
Based on the putative pathogenesis of the increased PT rates in recent years, Mehra et al. enumerate implications for the surveillance and prevention of PT that should be implemented (16). Pre-implant, patients who have experienced any episode of thromboembolism should undergo a thrombophilia workup. A critical point to be emphasized is that a meticulous history is essential during the pre-VAD work-up, since this will be the most fruitful avenue towards identifying patients with a prothrombotic tendency. As a result of this work-up, some patients may be denied VAD implantation, and others may have perhaps a more aggressive AC management. Patients with prior hematological disorders have been shown to have a worse outcome after VAD implantation, but the heterogeneity of the patient population in such studies makes it premature to advocate for or against LVAD implantation in these patients at present (35,36).
Given the time course of changes in AC regimens and the increase in PT rates, it is probably judicious to resume bridging with intravenous AC and aiming for a higher INR target of 2.0-3.0. For the HMII, downshifting in AC management seems to have preceded the increase in PT rates, although one would have expected the uptick to occur much earlier than 2013, given that this downshifting occurred at least two years earlier. For the HVAD, within the trial it was noted that there was initially a higher-than-expected PT rate, which prompted upshifting in the AC regimen. This latter change in management led to a decrease in PT rates. This data is further obfuscated by the superimposed change in the HVAD’s inflow design from polished titanium to sintered titanium. Despite this confusing picture, resuming heparin bridging and an uptitration to an INR target of 2.0-3.0 is prudent. If patients develop bleeding episodes on this regimen, it may need to be modified accordingly as is present practice. It is unfortunately exceedingly difficult to balance the risks of bleeding and PT in many of these patients.
A continually debated question is whether more scrupulous measurement of the patient’s coagulation management should be implemented for MCS patients. In a review of the topic, Gorlinger and colleagues have compared AC and antiplatelet management in VAD patients to “navigating between Scylla and Charybdis,” given the propensity for bleeding in these patients (37). The authors argue that conventional testing, including measurement of the INR, PTT, and platelet count, provides a very incomplete picture of the coagulation status of VAD patients. Hyperfibrinolysis, hypofibrinolysis, hypercoagulability due to tissue factor expression on circulating cells or increased clot firmness, and platelet aggregation cannot be adequately assessed by these limited means. They call for the increased use of whole blood viscoelastic tests, such as the thromboelastogram, and platelet function tests, such as aggregometry, to assess these patients. In addition, patients should be repeatedly tested over time, since there is significant flux in the hematological profile over time (37).
The jury remains out on this matter, but many centers have begun using such technology to assess their MCS patients (38-41). If bleeding or thrombotic complication rates are optimized by such testing, then it may be that these technologies can help to navigate the perilous waters between these competing complications for most centers (37).
Modulation of the AC and antiplatelet regimen should occur with changing clinical circumstances that connote a changing predisposition towards thromboembolism. For example, if a patient develops de novo atrial fibrillation, or is noted to have de novo clots in the aortic root or left ventricle, the patient’s AC and antiplatelet regimen should be uptitrated and bridging intravenous AC considered until the new therapeutic target is reached. As another example, if a patient were to develop a serious infection, this too should prompt at least a temporary uptitration of the regimen until the episode has cleared.
Hemolysis should be closely monitored with biweekly LDH levels, and further diagnostic and therapeutic management dictated by the ISHLT framework (to be explored in the following section). Mechanical factors should likewise be monitored over time. Changes in the angulation of the LVAD or pump pocket depth on chest X-ray (29) should prompt further investigations that may include measurement of hemolytic parameters, echocardiogram, and/or CT angiography.
Regardless of the etiology or presentation, if there is clinical suspicion of PT, workup and treatment should occur without delay. Although evidence is not available, it is reasonable to expect that earlier implementation of medical lytic or antithrombotic therapy will presumably be more successful—if it is to be successful at all (vide infra). Moreover, timely surgical intervention will likely lead to even better outcomes than are presently obtained.
Diagnosis
A multidisciplinary and multi-institutional ISHLT group devised an algorithm based on the best evidence for the diagnosis and management of PT, and this algorithm was published in 2013 (1). The algorithm is reproduced in Figure 11, and it displays a stepwise approach to guide the diagnosis and treatment of patients based on clinical presentation. The four clinical presentations recognized are as follows:
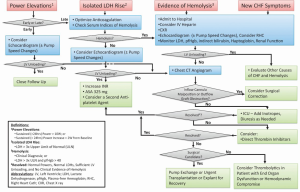
- Isolated power elevations;
- Isolated LDH rise;
- Evidence of hemolysis;
- New HF symptoms.
The approach is tailored to the presentation and further details can be found in the cited reference, which provides an outstanding “work-in-progress” framework for dealing with suspected PT (1).
The diagnosis of PT may be elusive because of the relative inaccessibility of the pump components to ultrasound and radiological examinations. One must often compile indirect information to arrive at the diagnosis. The history may elicit symptoms of HF, a change in urine color, evidence of noncompliance, interruption of AC, a clotting disorder, or a recent infection to suggest the diagnosis.
The physical exam may reveal signs of HF. An indispensable extension of the physical exam in LVAD patients is auscultation over the pump, including spectral analysis where available. A recent study prospectively characterized the acoustic spectra of HVADs by runtime fast Fourier transformation analysis (42). In comparing spectra of pumps with confirmed PT to those without, the group noted unique features of the sound peaks at certain frequencies in the PT patients that were diagnostic. Likewise, Hubbert et al. conducted a preliminary acoustic study of the HeartMate II device using a mock loop (43). In this study, changes to the acoustic fingerprint were noted during simulation of clots in the circuit.
With further refinement, this bedside methodology has the potential to detect PT at an earlier stage prior to onset of clinical signs, and will need further validation in future studies.
VAD interrogations may also provide useful information, and findings will vary dependent on the location within the pump of the PT. If the PT is located on the impeller itself, power spikes are frequent, and low flows with decrease PIs are accompaniments. If the PT is located upstream or downstream to the pump mechanism, both low power and low flow may be detected. It must be remembered that power is a surrogate for flow, and low flow states will often be reflected in low power consumption. Hence, PT can be associated with either high power spikes or power attenuation, dependent on its location.
Laboratory studies which support the diagnosis of PT are a rise in serum LDH, a drop in haptoglobin, a rise in plasma-free hemoglobin, a rise in bilirubin, or a rise in fibrin split products. Of all the laboratory studies, serum LDH appears to be the most reliable parameter for diagnosing actual or impending LVAD thrombosis (6,18,44). Interestingly, Bartoli et al. found that laboratory markers were even better at diagnosing LVAD thrombosis than either echocardiographic or pump parameters (45).
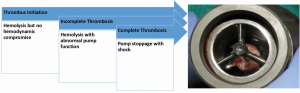
On numerous studies, hemolysis appears to be a harbinger of both PT as well as a poor prognosis (6,46,47). In the series, there was a distinct rise in serum LDH starting approximately one month prior to the diagnosis of CPT. Figure 12 depicts the theorized relationship between the LDH rise and the development of PT. Thrombus initiation transitions to incomplete thrombosis and finally—if not addressed—to complete thrombosis. LDH levels rise during the first two periods, while completed PT occurs in the last. In addition, the Columbia group found that a higher LDH at patient discharge following HMII implantation was associated with the later development of “non-mechanical” PT. In any event, a rising LDH level should be investigated as suggested in the algorithm in Figure 11.
The University of Louisville group has created a thrombosis risk index (TRI) comprised of INR, BNP, and LDH to act as surrogates for hypercoagulability, HF, and hemolysis, respectively (48). Retrospectively, the TRI would have triggered earlier recognition and therapy in most of their patients that developed PT. Prospective validation would be helpful in defining the role of this index, particularly for other centers.
Right heart catheterization shows data consistent with HF or cardiogenic shock. The arterial line will show greater pulsatility as the left ventricle remains full. The degree of enhanced pulsatility will correlate with underlying cardiac function.
Echocardiogram is essential to making the diagnosis, since it may show alteration in inflow velocities as well as the indices of a loaded heart. A ramp study can be performed, which will show failure of the LV to decrease in size as pump speed is increased (49). Estep’s group at Methodist Debakey showed that changes in three specific echocardiographic parameters—i.e., LVEDD, aortic valve opening time, and deceleration time of mitral inflow—during ramping up from 8,000 rpm through 11,000 rpm were reliably predictive in diagnosing PT (50-52).
Computerized tomography angiography (CTA) can be very helpful in diagnosing mechanical issues related to the LVAD that may predispose towards PT (53,54). Important positive findings include thrombus in the left ventricle, a dilated left ventricle, cannula malposition towards any of the left ventricular walls, outflow graft thrombus, kinking, or twisting, and lack of opacification of the outflow graft.
Left heart catheterization can be both diagnostic and therapeutic. Intraventricular contrast that fails to flow into the inflow cannula and fails to opacify the outflow graft are diagnostic of PT. Intraventricular thrombolytic agents may be considered once the diagnosis is made (vide infra).
Treatment
The algorithm discussed in the previous section also incorporates a stepwise approach to treatment options (Figure 11) (1).
Once the diagnosis of PT is established, there are a number of therapeutic options, but they fall into two general categories: medical management versus surgical management. The medical armamentarium includes heparin, GP IIb/IIIa inhibitors, and thrombolytics, either alone or in combination. Although isolated case reports have been published supporting the use of these agents (55-57), most larger volume clinical series have shown a less than satisfactory success rate. In Starling’s recent series of HMII thromboses, there was a 50% mortality rate for those patients treated medically, as opposed to a 5% mortality rate among those undergoing surgical therapy (6). While this data is impressive, it is of note that the vast majority of patients going to the operating room were in a relatively stable condition.
In Najjar’s HVAD series, there was a 50% success rate of medical therapy. Of those patients treated successfully, the most effective therapy was thrombolytic agents. Heparin alone was never successful. Of the fifteen patients who failed medical therapy, one died, 12 underwent device exchanges, and two underwent urgent transplantation. Hence, a medical approach does not preclude surgery if the former fails. However, all patients who underwent primary pump exchange survived, and a third of patients who underwent secondary pump exchange after failure of medical therapy died (7).
In a series from Duke, intraventricular thrombolytic therapy was performed in eight patients with PT. Of the eight, three were successfully treated, one required emergent LVAD exchange, one required urgent heart transplantation, and three died. Hence, the procedure was unsuccessful in over half of the patients, and most who were not successfully treated died (58).
Like thrombolytic therapy, treatment with GP IIb/IIIa agents has had variable success among different series. Tellor administered eptifibatide (integrilin) on 22 occasions to 17 patients in his series, and over half of these treatments included concomitant heparin. There was a high morbidity in this series. The median time from implantation until PT was fairly early at 47 days. There were significant bleeding episodes in half the administrations: there were four hemorrhagic strokes and five GI bleeds. Three patients (18%) underwent device exchange, and seven patients (41%) died. This series raises the question if the morbidity of medical therapy is worth its therapeutic efficacy (59). In a small series from Yale, four patients with PT were similarly treated with eptifibatide, but only one was successful. Two required LVAD exchange, and one patient died (60).
Hence, either thrombolytic or IIb/IIIa agents may be attempted in selected cases, but the chances of success are less than half. Some treatment failures may be treated by heart transplantation where short wait times are feasible or by LVAD exchange. A burning question remains: is anything lost by attempting medical therapy first? There is scant evidence to support attempting medical therapy first versus going directly to surgical therapy, but it is likely that patients who are in shock at the time of treatment have a poor prognosis regardless.
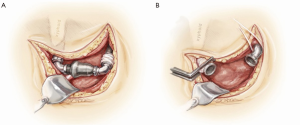
With the—at best—mixed success rate with medical therapy, it is prudent that urgent or emergent pump exchange be considered early for most patients. In Starling’s series, the operative mortality was 5% for patients undergoing pump exchange for PT. The salutary outcomes with surgery performed in a timely fashion, most via a less invasive subcostal approach, certainly would imply that a surgical approach be entertained (Figure 13).
One potential downside of a less invasive surgical approach is the possibility that the primary etiologic factor causing the PT may not reside in the pump body itself. If the main issue is either upstream or downstream to the pump body, it may be missed. Hence, one must be meticulous in attempting to exclude these possibilities with the appropriate preoperative diagnostic studies. If there is thrombus in the left ventricle, inflow cannula, or outflow graft, or if there is a mechanical problem with the inflow cannula, outflow graft, or outflow anastomosis, this approach should not be used. Instead, patients should undergo conventional redo sternotomy, where all elements of the LVAD and heart are accessible.
With the above caveats in mind, however, a subcostal approach is feasible for most patients (Figure 13) (61). For this approach, cardiopulmonary bypass (CPB) is undertaken with either axillary or femoral arterial cannulation and femoral venous cannulation. Prior to cannulation, the pump body, inflow elbow, and outflow elbow, bend relief, and proximal graft are exposed, followed by heparinization and cannulation. The new replacement LVAD is brought up to the field and prepared. The driveline is brought out through the skin, most commonly on the side opposite where the present exit site is located. A carbon dioxide line is secured at the superior skin edge to insufflate the field. The bend relief is disconnected to expose the connection between the outflow elbow and the outflow graft. Once CPB is instituted, the outflow graft is clamped and the LVAD is turned off. A supplied wrench is used to disconnect both the inflow elbow and the outflow graft from the pump, and subsequently the pump is examined and set aside. CPB is turned down momentarily, and the graft clamp released to backflush the graft and ensure that there are no clots in the outflow graft. Endoscopy of the inflow cannula can be performed, as discussed below. The new pump is prepared and connected to the inflow and outflow regions with the wrench. A 19 gauge needle is inserted into the outflow graft proximal to the clamp, and the pump is activated. Ventilations are resumed, and the heart is allowed to fill. Deairing is carried out, and the patient is weaned from CPB as the pump is uptitrated. Deairing is a challenge in these patients, so TEE guidance is crucial in determining when all the intracardiac air has been adequately removed. The clamp is progressively removed from the graft so as to minimize the influx of trapped air from the LVAD to the aorta.
A potentially useful adjunct for the subcostal replacement of an LVAD was reported upon by the University of Rochester team. “VADoscopy” was performed with a 5 French 30 cm flexible endoscope (Karl Starz Flex-X, Germany) that is placed in the inflow elbow once the intraventricular portion of the inflow cannula is occluded with a 22 Fr Fogarty balloon (62). The inflow cannula and elbow can then be visualized via the scope to ensure there is no further debris or thrombus in those portions of the VAD.
Moazami et al. described HMII replacements in 72 patients who underwent 79 replacements. The operative approach included a sternotomy in at least 70% of the cases, and a subcostal approach in at least 26%. Seventy-seven replacements were performed during the chronic phase, and two were performed emergently. For the whole series, operative mortality was 6.5%, and no patients who underwent the subcostal approach died (63).
While surgical therapy appears to be associated with good postoperative outcomes, the long-term outlook for patients operated upon for PT are inferior to that for patients undergoing primary pump placement. The INTERMACS database showed a significant decrease in two year survival in patients who required device exchange as compared to survival after initial implantation (56% vs. 69%, P<0.0001) (8). Whether this can be improved upon by earlier diagnosis and intervention remains to be seen.
Summary and conclusions
PT is thus a complex, challenging, elusive, and threatening problem—truly a “riddle wrapped in a mystery inside an enigma.” Its etiology is multifactorial and definite management changes are indicated in light of the data we have at present, including resuming bridging patients with heparin or alternative agents and aiming for higher target INRs. The ISHLT algorithm for the diagnosis and management of PT is a very valuable guide. At this juncture, more studies are indicated to further evaluate therapeutic alternatives in the management of VAD patients and how to negotiate the “Scylla and Charibdis” of competing morbidities.
Nonetheless, the rising incidence of PT needs to be understood against the background of the overall great strides in MCS. The riddle has yet to be unraveled, the mystery solved, and the enigma deciphered—but progress is being made. Once the PT problem has been controlled, only then can we reasonably expect to contemplate extending MCS to the less ill patient population identified by the ROADMAP and REVIVE-IT studies.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [PubMed]
- CDC. eds. The State of Aging and Health in America 2013. Atlanta, GA: Services USDoHaH, 2013.
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant 2011;30:402-7. [PubMed]
- The Evaluation of VAD InterVEntion Before Inotropic Therapy (REVIVE-IT) Trial [Internet]. Available online: www.ClinicalTrials.Gov. 2014.
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33:12-22. [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Lahpor J, Khaghani A, Hetzer R, et al. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardiothorac Surg 2010;37:357-61. [PubMed]
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [PubMed]
- Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant 2010;29:616-24. [PubMed]
- Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant 2009;28:881-7. [PubMed]
- Mehra MR, Stewart GC, Uber PA. The vexing problem of thrombosis in long-term mechanical circulatory support. J Heart Lung Transplant 2014;33:1-11. [PubMed]
- Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circulation Heart Failure 2012;5:241-8. [PubMed]
- Uriel N, Han J, Morrison KA, et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant 2014;33:51-9. [PubMed]
- Wang JX, Lee EH, Bonde P. Over 400% Increase in LVAD Thrombosis Reported to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) Database from 2010 to 2012. J Heart Lung Transplant 2014;33:S9-10.
- Bartoli CR, Ailawadi G, Kern JA. Diagnosis, nonsurgical management, and prevention of LVAD thrombosis. J Card Surg 2014;29:83-94. [PubMed]
- Ledford ID, Labedi M, Kfoury AG, et al. 235 Thrombus within the HeartMate II Left Ventricular Assist Device (LVAD): Are All Clots Created Equal? J Heart Lung Transplant 2014;31:S85-6.
- Marsden AL, Bazilevs Y, Long CC, et al. Recent advances in computational methodology for simulation of mechanical circulatory assist devices. Wiley Interdiscip Rev Syst Biol Med 2014;6:169-88. [PubMed]
- Chiu WC, Slepian MJ, Bluestein D. Thrombus formation patterns in the Heartmate II ventricular assist device: Clinical observations can be predicted by numerical simulations. ASAIO J 2014;60:237-40. [PubMed]
- Birschmann I, Dittrich M, Eller T, et al. Ambient hemolysis and activation of coagulation is different between HeartMate II and HeartWare left ventricular assist devices. J Heart Lung Transplant 2014;33:80-7. [PubMed]
- Stulak JM, Deo S, Schirger J, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg 2013;96:2161-7. [PubMed]
- Wu L, Weng YG, Dong NG, et al. Outcomes of HeartWare Ventricular Assist System support in 141 patients: a single-centre experience. Eur J Cardiothorac Surg 2013;44:139-45. [PubMed]
- Warkentin TE, Greinacher A, Koster A. Heparin-induced thrombocytopenia in patients with ventricular assist devices: are new prevention strategies required? Ann Thorac Surg 2009;87:1633-40. [PubMed]
- Pappalardo F, Scandroglio AM, Potapov E, et al. Argatroban anticoagulation for heparin induced thrombocytopenia in patients with ventricular assist devices. Minerva Anestesiol 2012;78:330-5. [PubMed]
- Taghavi S, Ward C, Jayarajan SN, et al. Surgical technique influences HeartMate II left ventricular assist device thrombosis. Ann Thorac Surg 2013;96:1259-65. [PubMed]
- Shah MR, Naftel DC, Miller MA, et al. Thrombotic Complications Increase after Percutaneous Site/Pocket Infection in Patients with Left Ventricular Assist Devices: An INTERMACS Analysis. J Heart Lung Transplant 2003;32:S84-5.
- Whitson BA, Eckman P, Kamdar F, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 2014;97:2097-103. [PubMed]
- Stulak JM, Lee D, Haft JW, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant 2014;33:60-4. [PubMed]
- Jennings DL, Jacob M, Chopra A, et al. Safety of Anticoagulation Reversal in Patients Supported with Continuous-flow Left-ventricular Assist Devices. ASAIO J 2014. [Epub ahead of print]. [PubMed]
- Wong K, Samaroo G, Ling I, et al. Intraventricular flow patterns and stasis in the LVAD-assisted heart. J Biomech 2014;47:1485-94. [PubMed]
- Fried J, Levin AP, Mody KM, et al. Prior hematologic conditions carry a high morbidity and mortality in patients supported with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2014. [Epub ahead of print]. [PubMed]
- Connors JM. Hematologic Disorders and Continuous Flow-Left Ventricular Assist Devices. J Heart Lung Transplant. 2014. [Epub ahead of print]. [PubMed]
- Görlinger K, Bergmann L, Dirkmann D. Coagulation management in patients undergoing mechanical circulatory support. Best Pract Res Clin Anaesthesiol 2012;26:179-98. [PubMed]
- Fries D, Innerhofer P, Streif W, et al. Coagulation monitoring and management of anticoagulation during cardiac assist device support. Ann Thorac Surg 2003;76:1593-7. [PubMed]
- Majeed F, Kop WJ, Poston RS, et al. Prospective, observational study of antiplatelet and coagulation biomarkers as predictors of thromboembolic events after implantation of ventricular assist devices. Nat Clin Pract Cardiovasc Med 2009;6:147-57. [PubMed]
- Ensor CR, Cahoon WD, Crouch MA, et al. Antithrombotic therapy for the CardioWest temporary total artificial heart. Tex Heart Inst J 2010;37:149-58. [PubMed]
- Sun W, Jeleniowski K, Zhao X, et al. Thromboelastography (TEG)-Based Algorithm Reduces Blood Product Utilization in Patients Undergoing VAD Implant. J Card Surg 2014;29:238-43. [PubMed]
- Kaufmann F, Hormandinger C, Stepanenko A, et al. Acoustic spectral analysis for determining pump thrombosis in rotary blood pumps. ASAIO J 2014;60:502-7. [PubMed]
- Hubbert L, Sundbom P, Loebe M, et al. Acoustic analysis of a mechanical circulatory support. Artificial Organs 2014;38:593-8. [PubMed]
- Shah P, Mehta VM, Cowger JA, et al. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant 2014;33:102-4. [PubMed]
- Bartoli CR, Ghotra AS, Pachika AR, et al. Hematologic Markers Better Predict Left Ventricular Assist Device Thrombosis than Echocardiographic or Pump Parameters. Thorac Cardiovasc Surg 2014;62:414-8. [PubMed]
- Ravichandran AK, Parker J, Novak E, et al. Hemolysis in left ventricular assist device: a retrospective analysis of outcomes. J Heart Lung Transplant 2014;33:44-50. [PubMed]
- Cowger JA, Romano MA, Shah P, et al. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant 2014;33:35-43. [PubMed]
- Trivedi JR, Sobieski MA, Schwartz S, et al. Novel thrombosis risk index as predictor of left ventricular assist device thrombosis. ASAIO J 2013;59:380-3. [PubMed]
- Fine NM, Topilsky Y, Oh JK, et al. Role of echocardiography in patients with intravascular hemolysis due to suspected continuous-flow LVAD thrombosis. JACC Cardiovasc Imaging 2013;6:1129-40. [PubMed]
- Estep JD, Vivo RP, Cordero-Reyes AM, et al. A simplified echocardiographic technique for detecting continuous-flow left ventricular assist device malfunction due to pump thrombosis. J Heart Lung Transplant 2014;33:575-86. [PubMed]
- Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol 2012;60:1764-75. [PubMed]
- Kato TS, Colombo PC, Nahumi N, et al. Value of serial echo-guided ramp studies in a patient with suspicion of device thrombosis after left ventricular assist device implantation. Echocardiography 2014;31:E5-9. [PubMed]
- Acharya D, Singh S, Tallaj JA, et al. Use of gated cardiac computed tomography angiography in the assessment of left ventricular assist device dysfunction. ASAIO J 2011;57:32-7. [PubMed]
- Mishkin JD, Enriquez JR, Meyer DM, et al. Utilization of cardiac computed tomography angiography for the diagnosis of left ventricular assist device thrombosis. Circulation Heart Failure 2012;5:e27-9. [PubMed]
- Thenappan T, Anderson AS, Jeevanadham V, et al. Treatment of left ventricular assist device thrombosis with extended catheter-directed intraventricular thrombolytic therapy. Circulation Heart Failure 2013;6:e27-9. [PubMed]
- Jabbar AA, Yau R, Frazier OH, et al. Direct thrombolytic therapy for thrombosis of a centrifugal flow left ventricular assist device. ASAIO J 2013;59:530-2. [PubMed]
- Kamouh A, John R, Eckman P. Successful treatment of early thrombosis of HeartWare left ventricular assist device with intraventricular thrombolytics. Ann Thorac Surg 2012;94:281-3. [PubMed]
- Schlendorf K, Patel CB, Gehrig T, et al. Thrombolytic therapy for thrombosis of continuous flow ventricular assist devices. J Card Fail 2014;20:91-7. [PubMed]
- Tellor BR, Smith JR, Prasad SM, et al. The use of eptifibatide for suspected pump thrombus or thrombosis in patients with left ventricular assist devices. J Heart Lung Transplant 2014;33:94-101. [PubMed]
- Bellumkonda L, Subrahmanyan L, Jacoby D, et al. Left ventricular assist device pump thrombosis: is there a role for glycoprotein IIb/IIIa inhibitors? ASAIO J 2014;60:134-6. [PubMed]
- Ota T, Yerebakan H, Akashi H, et al. Continuous-flow left ventricular assist device exchange: clinical outcomes. J Heart Lung Transplant 2014;33:65-70. [PubMed]
- Cheng A, Swartz MF, Massey HT. VADoscopy: a novel intraoperative technique to evaluate HeartMate II left ventricular assist device inflow obstruction and thrombosis. ASAIO J 2013;59:671-4. [PubMed]
- Moazami N, Steffen RJ, Naka Y, et al. Lessons Learned From the First Fully Magnetically Levitated Centrifugal LVAD Trial in the United States: The DuraHeart Trial. Ann Thorac Surg 2014;98:541-7. [PubMed]