Left ventricular assist device outflow graft: alternative sites
Ventricular assist device (VAD) implantation has become a well-accepted option to treat patients with end-stage heart disease, particularly due to the lack of the available heart donors and the increasing number of patients presenting with end-stage heart disease (1,2). Initially, utilization of VAD was indicated for patients waiting for heart transplantation [bridge to transplantation (BTT)] (3) and for patients with a temporary need for circulatory support (bridge to recovery) (4).
With the technical advancement and development of more robust devices, the indication for VAD implantation has broadened to include patients who are not candidates for transplantation (destination therapy) (5) as well as patients in situations of extreme hemodynamic instability (bridge to decision) (6).
This paper aims to describe three alternative approaches for the left ventricular assist device (LVAD) outflow graft placement.
Technique
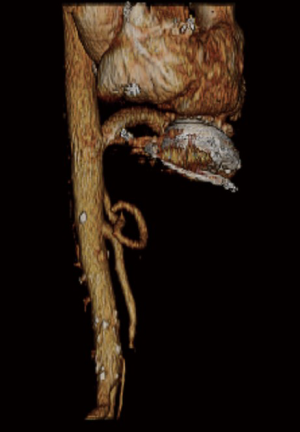
Outflow graft to the supraceliac abdominal aorta
This is a 51-year-old female patient who most likely developed end-stage cardiomyopathy secondary to adriamycin toxicity. She has a history of right-sided breast cancer, which was treated with multiple breast lumpectomies followed by completion modified radical mastectomy and breast reconstruction. Additionally, she also received both adriamycin-based chemotherapy (four cycles) and radiation therapy to the chest field. Her past medical history also included hypertension, hepatic dysfunction secondary to congestive hepatopathy, right-sided pleural effusion and cardiac cachexia. After three cycles of adriamycin, her echocardiogram showed an ejection fraction (EF) of 65%. She proceeded to receive the 4th cycle of adriamycin as well as 10 cycles of chest radiation therapy. Later on, the patient developed symptoms of worsening dyspnea, orthopnea, and paroxysmal nocturnal dyspnea. The interval echocardiogram revealed an EF of 30%. She had multiple hospitalizations and was started on intravenous (IV) inotropes and diuretic therapy with minimal clinical improvement. She was then transferred to our institute for consideration for mechanical circulatory support. The option of HeartWare left ventricular assist device (HVAD) (HeartWare International, Inc., Framingham, Massachusetts, USA) implantation, as BTT, was discussed with the patient. Due to her history, the decision was made to employ the sternotomy-sparing left subcostal incision and utilize the supraceliac abdominal aorta as a route to the LVAD outflow graft.
The left subcostal incision was performed and extra-peritoneal exposure of the supraceliac abdominal aorta was achieved. Both the left common femoral vein and artery were exposed. The left diaphragm was divided to expose the heart. The patient was fully heparinized, and after ensuring active clotting time (ACT) >500, standard femoral cannulation for cardiopulmonary bypass was performed. The HVAD was implanted on the diaphragmatic surface of the left ventricle (LV) and the outflow graft was trimmed to reach the supraceliac abdominal aorta. A side-biting clamp was applied onto the supraceliac abdominal aorta. The outflow graft to the supraceliac abdominal aorta anastomosis was fashioned in an end-to-side configuration (Figure 1). The driveline was tunneled through the left anterior abdominal wall and connected to the controller. The tunnel was created prior to heparin administration. The outflow graft was de-aired and the pump was turned on. The patient was successfully weaned from the cardiopulmonary bypass and tolerated the procedure very well.
Outflow graft to the innominate artery
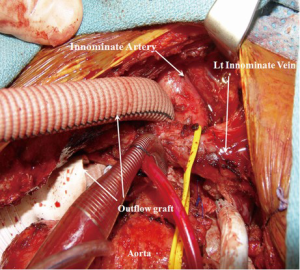
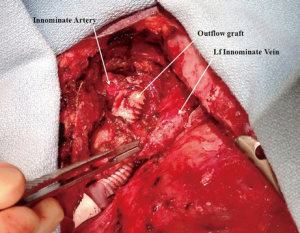
A 23-year-old African American female patient with a history of complex congenital valvular abnormalities and non-compaction cardiomyopathy presented with decompensated heart failure. She had four previous sternotomies. Her surgeries included resection of a sub-aortic membrane and LV myomectomy in 1996, Ross procedure in 2003, mechanical aortic valve implantation in March 2010 and a subsequent aortic root replacement with a 25-mm Medtronic freestyle porcine aortic root bioprosthesis (Medtronic Inc., Minneapolis, Minnesota, USA) in August 2010. She also had a past medical history of automated implantable cardiac defibrillator (AICD) implantation, embolic infarct to the right kidney, deep venous thrombosis, pulmonary embolism and minor stroke with no residual motor and sensory deficit. Her echocardiography revealed a left ventricular EF of <30%, severe pulmonary regurgitation and severe tricuspid regurgitation. Computed tomography (CT) angiography of chest revealed heavy calcification of both the ascending aorta and the pulmonary homograft. The patient was evaluated for HVAD (HeartWare International, Inc.) implantation as a BTT.
Femoral artery and vein were accessed to establish total cardiopulmonary bypass circuit. After the fifth repeat median sternotomy, the superior vena caval cannulation was performed and total cardiopulmonary bypass was initiated. Both cavae were snared to achieve complete isolation of right atrium. The patient underwent pulmonary valve replacement utilizing a 23-mm St. Jude Medical Epic bioprosthesis (St Jude Medical Inc, St Paul, Minnesota, USA) and reconstruction of the right ventricle outflow tract with a bovine pericardial patch (Vascuguard; Synovis Life Technologies, St. Paul, Minnesota, USA). The tricuspid valvuloplasty was then performed using a 28-mm Edwards MC3 partial annuloplasty ring (Edwards Lifesciences, Irvine, California, USA). Next, the HVAD was implanted on the diaphragmatic surface of the LV. As the ascending aorta was heavily calcified, the innominate artery was selected for the outflow graft anastomosis. The outflow graft was positioned rostral to the inferior vena cava (IVC), tunneled along the right side of the heart and passed rostral to the left innominate vein to reach the innominate artery. The outflow graft was reinforced with a ring-reinforced Gore-Tex graft (W.L. Gore and Associates, Inc., Flagstaff, Arizona, USA). After applying a side-biting clamp to the innominate artery, the outflow graft was anastomosed in an end-to-side fashion (Figures 2,3). The driveline was tunneled through the anterior abdominal wall and connected to the controller. The tunnel was created prior to heparin administration. The outflow graft was de-aired and the patient was successfully weaned from the cardiopulmonary bypass. The patient tolerated the procedure very well.
Outflow graft to the left axillary artery
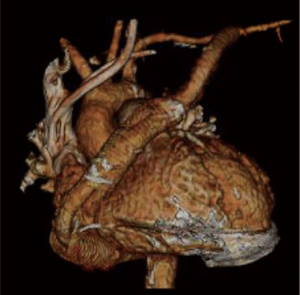
A 51-year-old female patient with a history of ischemic cardiomyopathy presented with symptoms of congestive heart failure. Her past medical history was notable for coronary artery disease, hypertension, multiple percutaneous coronary interventions, pulmonary hypertension, chronic kidney disease, obesity, dyslipidemia and anemia. The echocardiography revealed a severely dilated LV with an EF of 20%. The patient had a history of HVAD (HeartWare International, Inc.) implantation as a BTT. Seven months later, she developed methicillin-resistant staphylococcus aureus bacteremia with radiological evidence of peri-anastomotic abscess and pseudoaneurysm formation at the outflow graft-aortic anastomosis site. The appropriate antibiotic therapy was initiated with no clinical improvement. Hence, the surgical drainage of the collection, repair of pseudoaneurysm and repositioning of the outflow conduit to the left axillary artery was planned.
The patient underwent the repeat median sternotomy utilizing femoro-femoral cannulation for the cardiopulmonary bypass. A brief period of deep hypothermic circulatory arrest was utilized to repair the ascending aortic pseudoaneurysm using a bovine pericardial patch (Vascuguard; Synovis Life Technologies). The HVAD outflow graft was resected leaving only proximal 10 cm attached to the pump. The abscess cavity was debrided and all the infected material was completely removed. The left axillary artery was exposed and a 10 mm Gelweave graft (Vascutek/Terumo, Renfrewshire, Scotland) was anastomosed to the left axillary artery. The graft was then tunneled through the left 2nd intercostal space to reach the left chest cavity, and a graft-to-graft anastomosis was performed in the mediastinum. A ring-reinforced Gore-Tex graft (W.L. Gore and Associates, Inc.) was used to cover the 10 mm tunneled graft as it passes through the left 2nd intercostal space (Figure 4). The patient was successfully weaned from the cardiopulmonary bypass and tolerated the procedure well. Postoperatively, the patient was followed up using the serial speed-change echocardiography and Doppler ultrasound of the outflow graft. The left upper extremity had been clinically examined regularly with minimal extremity swelling and no evidence of overflow or malperfusion.
Comments
Median sternotomy is the standard approach for LVAD implantation. The inflow cannula is secured to the LV apex and the outflow graft attached to the ascending aorta. Left lateral thoracotomy had been described as an alternative approach for the LVAD implantation with securing of the inflow cannula to the LV apex and the outflow graft to the descending thoracic aorta. Recently, a less invasive approach has been described for HeartMate II (Thoratec, Pleasanton, California, USA) implantation through a left subcostal incision for attachment of the inflow cannula to the LV apex, combined with a right mini-thoracotomy for anastomosis of the outflow graft to the ascending aorta (7). Riebandt et al. (8) described a minimally invasive Thoratec HeartMate II (Thoratec) implantation in the setting of severe ascending thoracic aortic calcification. They utilized the left subcostal approach to secure the pump to the LV apex and position it in the preperitoneal pocket. The outflow graft was tunneled through the diaphragm to reach the right pleural cavity, then passed through the right 2nd intercostal space and anastomosed to the right subclavian artery.
In this report, we describe three alternative approaches to attach the LVAD outflow graft to different positions in the arterial system other than the ascending aorta. HVAD (HeartWare International, Inc.) was used in our report.
In the first approach, the patient had several breast surgeries and chest wall radiation for the treatment of breast cancer. The left subcostal approach had been employed to avoid the hostile chest wall. This incision provided us with an excellent exposure to both the heart and the supraceliac abdominal aorta. The pump was secured to the diaphragmatic surface of the LV and the outflow graft was trimmed and cut to the length to reach the supraceliac abdominal aorta. This sternotomy-sparing approach was very helpful to accomplish the LVAD implantation while avoiding the risks of bleeding, infection and potential of impaired healing with median sternotomy. The postoperative echocardiography and imaging studies showed good positioning of the LVAD, excellent unloading of the LV and an optimal flow into the outflow graft.
In the second approach, a repeat median sternotomy approach was utilized as the patient required concomitant procedures to address the severe pulmonary and tricuspid regurgitation in addition to end-stage heart failure. Given the heavily calcified ascending aorta, the innominate artery was selected as an alternative site for the LVAD outflow graft anastomosis. The outflow graft was supported with a ring-reinforced Gore-Tex graft (W.L. Gore and Associates, Inc.) and passed rostral to both IVC and innominate vein to protect the graft and position it away from the back of the sternum. The anastomosis of the outflow graft to the innominate artery was achieved without interrupting the cerebral blood flow by applying a side-biting clamp.
In the third technique, the patient had previous HVAD implantation and developed an abscess with a pseudoaneurysm at the outflow graft-to-aortic anastomosis site. The drainage of the abscess and repair of the pseudoaneurysm was an urgent surgical intervention. The complete resection and debridement of infected material and rerouting the LVAD outflow graft was paramount to completely eliminate the infection and avoid the risk of reinfection. The left axillary artery was considered an optimal location for the outflow graft anastomosis. The LVAD outflow graft was tunneled through the left pleural cavity due to the fact that the infected field was in close proximity to the right pleural cavity. The left 2nd intercostal space was wide enough to accommodate the outflow graft, which was further protected with a ring-reinforced Gore-Tex (W.L. Gore and Associates, Inc.) graft as it passes through the intercostal space. Postoperatively, the patient showed a consistent good flow in the outflow graft and insignificant swelling of the left upper extremity. Additionally, the preoperative infection was completely eliminated.
From our report of surgical techniques, we conclude that supraceliac abdominal aorta, innominate artery and left axillary artery and possibly right subclavian artery should be considered as potential sites for the LVAD outflow graft anastomosis in the setting of a heavily calcified ascending aorta or a hostile chest wall/mediastinal cavity. Furthermore, the outflow graft can be tunneled through either pleural cavity to avoid the hostile field. Although the described alternative approaches are safe and viable options, we continue to recommend utilizing these alternative approaches only in selected patients with significantly higher risks and hazards with the standard surgical approach.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Thunberg CA, Gaitan BD, Arabia FA, et al. Ventricular assist devices today and tomorrow. J Cardiothorac Vasc Anesth 2010;24:656-80. [PubMed]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011;123:e18-e209. [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 2006;355:1873-84. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Ziemba EA, John R. Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg 2010;25:425-33. [PubMed]
- Anyanwu AC. Technique for less invasive implantation of Heartmate II left ventricular assist device without median sternotomy. Semin Thorac Cardiovasc Surg 2011;23:241-4. [PubMed]
- Riebandt J, Sandner S, Mahr S, et al. Minimally invasive thoratec Heartmate II implantation in the setting of severe thoracic aortic calcification. Ann Thorac Surg 2013;96:1094-6. [PubMed]





