Biventricular VAD versus LVAD for right heart failure
Introduction
Right ventricular failure (RVF) in the era of left ventricular assist device (LVAD) therapy remains a significant problem. Approximately 6% to 10% of patients with an LVAD will require the implantation of a right ventricular assist device (RVAD) (1) with an additional 15% to 20% requiring prolonged inotropic support for RVF (2,3). Several mechanisms may contribute to RVF post LVAD implantation, most importantly the unloading of the left ventricle and resultant loss of septal contribution to right ventricular (RV) function. In addition, perioperative factors such as myocardial ischemia can further compromise a vulnerable right ventricle. As these factors may lead to rescue implantation of an RVAD, which is associated with increased mortality (4), research should focus on identifying patients that would benefit from preemptive implantation of an RVAD. Here we highlight recent advances in the field, focusing on risk stratification scores, the use of pulmonary vasodilators, the use of biventricular assist devices (BIVAD) versus a total artificial heart (TAH), and the use of a temporary RVAD (tRVAD). We also briefly present recent data on right heart recovery post LVAD using tRVAD support.
Highlights of novel risk scores for RVF post-LVAD
There has been much interest in refining RVF risk scores in the setting of newer imaging modalities and continuous flow devices. Three recent studies on risk stratification for RVF post LVAD stand out. The articles highlight the utility of echocardiographic derived RV strain indices coupled with markers of RV function to provide a superior means of scoring and predicting RVF. These recent scores build on previous methods that combine parameters of right heart function (systolic dysfunction or tricuspid regurgitation severity); severity of heart failure (cardiac index, temporary mechanical support); end organ dysfunction (hepatic, pulmonary and renal); or other demographic or clinical aspects (Table 1) (5-10). Grant et al. (11) examined RV function in 117 patients undergoing continuous flow LVAD implantation. Incorporating RV longitudinal strain, the authors demonstrated that RV free wall strain <9.6% was incremental to the Michigan score (8) in stratifying the risk for RV failure post-LVAD implantation: the area under the curve (AUC) of the Michigan score increased from 0.66 to 0.77 (P<0.01) when augmented with RV strain. Finally, the authors noted that neither RV fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE) nor LV-RV geometry were independent predictors of RVF.
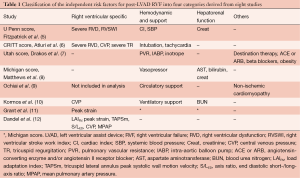
Full table
Dandel et al. (12) focused on echocardiographic measures of the coupling between RV geometry and afterload, which they referred to as the RV load adaptation index (LAIRV). The LAIRV is derived from the velocity time integral (VTI) of tricuspid regurgitation (cm) multiplied by the end diastolic (ED) RV long axis length (cm) divided by RV ED area (cm2). Accordingly, patients with aberrant geometry and low afterload have low LAIRV values, whereas patients with preserved RV geometry and maintained afterload have a higher LAIRV. In 205 patients, LAIRV predicted RV failure with an AUC of 0.906. As well, authors confirmed RV longitudinal strain as a relevant parameter for preoperative risk stratification.
Atluri et al. (6) devised a new RV risk score (the CRITT score) during the current era of continuous flow devices. The CRITT score includes the five following parameters: central venous pressure >15 mmHg (C), severe RV dysfunction (R), preoperative intubation (I), severe tricuspid regurgitation (T) and tachycardia defined by heart rate >100 bpm (T). The CRITT score attributes a risk varying from 0 to 5, with scores of 1 to 2 representing a negative predictive value for RVAD of 93%. The score is in part an extension of the previously published Penn score (5) but has the advantage of incorporating routinely obtained echocardiographic information, imparting the CRITT score with a superior discrimination for RV failure compared to the Michigan score.
Taken together, these recent scoring systems highlight the power and utility of incorporating echocardiographic information to augment invasive hemodynamic and biomarker measurements. While further validation is required, these scoring approaches offer valuable insight into providing a preemptive strategy for RVAD implantation in high risk cohorts.
Highlights of the use of pulmonary vasodilators in patients at risk of RVF
Inhaled nitric oxide or oral sildenafil are often used either peri-operatively or after LVAD implantation in patients deemed high risk for RVF. In a small prospective randomized study, Potapov et al. (13) demonstrated that the use of inhaled nitric oxide after LVAD implantation did not decrease the incidence of RVF. In a recent, open-labeled controlled trial by Tedford et al. (14), sildenafil decreased pulmonary vascular resistance and improved hemodynamic indices of right heart function after LVAD implantation when compared to the control group. Although these studies show a desired measurable physiologic effect, the use of either nitric oxide or phosphodiesterase inhibitors does not yet demonstrate a clear benefit with regards to preventing RVF.
Highlights of the surgical management of patients at risk of RVF
Surgical management of patients at risk of RVF is challenging and lends itself to two crucial questions: (I) whether a TAH should be considered instead of a BIVAD; and (II) what is the role of a tRVAD. Kirsch et al. (15) recently reported a large multicenter experience in France comparing the use of BIVAD support and a TAH. There was no significant difference between groups in survival while on support. The main difference was the higher incidence of stroke in the patients with BIVAD therapy, with a trend toward increased survival in the TAH group after 90 days attributed to a decreased incidence of neurologic events. However, not all patients at risk of RVF should be considered for permanent biventricular support.
Advances in the field of tRVADs have allowed devices to be removed within days or weeks as a bridge to RV recovery, or switched to long-term RVAD support if needed. These advances include the use of right atrium to pulmonary artery extracorporeal life support that may be implanted perioperatively and removed percutaneously (16,17). Delayed or unplanned implantation of a tRVAD proves to be an important risk factor for mortality in LVAD recipients (5). Moreover, Takeda et al. (18) highlighted the important prognostic value of RV recovery after unplanned tRVAD implantation. In their experience, patients not weaned from unplanned-tRVAD had a 6-month actuarial survival of 13%, whereas patients weaned from unplanned-tRVAD and those who underwent planned BIVAD implantation had a significantly higher survival (62% and 75%, respectively). This study shows that conditions leading to unplanned RVAD support may alter possibilities from RV recovery that significantly decrease early survival after LVAD implantation. Future studies should focus on recognition of perioperative factors impairing RV function and recovery after LVAD implantation.
Other studies demonstrate the potential benefit of early tRVAD use to avoid unplanned RVAD implantation. Lazar et al. (19) demonstrated that planned tRVAD implantation in low risk LVAD recipients was a safe approach with a high rate of tRVAD weaning (91.1%). Their results also showed that patients successfully weaned from tRVAD support had similar in-hospital mortality to those requiring isolated left ventricular support.
Conclusions
This review highlights recent advances in the preoperative risk stratification of RVF in patients requiring left ventricular mechanical circulatory support. Such stratification aims to guide planned surgical therapy for RVF including tRVAD, BiVAD or TAH implantation in order to decrease postoperative RVF and mortality associated with rescue therapy. Future studies should validate and improve these models, allowing us to better predict who will need biventricular support, and whether that support should be considered as a bridge to RV recovery, or as a destination RV support therapy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 2013;32:141-56. [PubMed]
- Patlolla B, Beygui R, Haddad F. Right-ventricular failure following left ventricle assist device implantation. Curr Opin Cardiol 2013;28:223-33. [PubMed]
- Haddad F, Couture P, Tousignant C, et al. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg 2009;108:422-33. [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hiesinger W, et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg 2009;137:971-7. [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hsu VM, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 2008;27:1286-92. [PubMed]
- Atluri P, Goldstone AB, Fairman AS, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg 2013;96:857-63; discussion 863-4. [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [PubMed]
- Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation 2002;106:I198-202. [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [PubMed]
- Grant AD, Smedira NG, Starling RC, et al. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 2012;60:521-8. [PubMed]
- Dandel M, Potapov E, Krabatsch T, et al. Load dependency of right ventricular performance is a major factor to be considered in decision making before ventricular assist device implantation. Circulation 2013;128:S14-23. [PubMed]
- Potapov E, Meyer D, Swaminathan M, et al. Inhaled nitric oxide after left ventricular assist device implantation: a prospective, randomized, double-blind, multicenter, placebo-controlled trial. J Heart Lung Transplant 2011;30:870-8. [PubMed]
- Tedford RJ, Hemnes AR, Russell SD, et al. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail 2008;1:213-9. [PubMed]
- Kirsch M, Mazzucotelli JP, Roussel JC, et al. Survival after biventricular mechanical circulatory support: does the type of device matter? J Heart Lung Transplant 2012;31:501-8. [PubMed]
- Saito S, Sakaguchi T, Miyagawa S, et al. Recovery of right heart function with temporary right ventricular assist using a centrifugal pump in patients with severe biventricular failure. J Heart Lung Transplant 2012;31:858-64. [PubMed]
- Loforte A, Stepanenko A, Potapov EV, et al. Temporary right ventricular mechanical support in high-risk left ventricular assist device recipients versus permanent biventricular or total artificial heart support. Artif Organs 2013;37:523-30. [PubMed]
- Takeda K, Naka Y, Yang JA, et al. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant 2014;33:141-8. [PubMed]
- Lazar JF, Swartz MF, Schiralli MP, et al. Survival after left ventricular assist device with and without temporary right ventricular support. Ann Thorac Surg 2013;96:2155-9. [PubMed]





