Impact of reverse remodeling on cardiac function
Introduction
Left ventricular assist devices (LVADs) have become the standard of care for treatment of patients with refractory end-stage heart failure (1-5). An LVAD results in decompression of the left ventricle, which promotes reverse remodeling, as manifested by decreased ventricular size, decreased mitral and tricuspid valve regurgitation and improved ventricular function. This process may play an important role in inducing left ventricular recovery with eventual explantation of the device.
Right ventricular function and right-sided hemodynamics are also improved with LVAD therapy. Along with unloading of the right ventricle, there is a substantial reduction in central venous pressure (CVP), pulmonary artery pressure (PAP) and tricuspid regurgitation (TR).
Consistent with the macroscopic changes seen on echocardiography as well as intracardiac pressures measured on right heart catheterizations, there are microscopic changes in the heart that are consistent with reverse remodeling after placement of an LVAD. These microscopic changes include changes in myocyte size, regression of fibrosis, cellular hypertrophy, collagen content, gene expression and various biomarkers of recovery, such as cardiac tumor necrosis factor-alpha (6-10).
Methods
We retrospectively analyzed our institutional experience with 100 consecutive CF LVADs. This included both HeartMate II (HM II) LVAD (Thoratec Corp., Pleasanton, CA, USA) recipients as well as HVAD (HeartWare Inc., Framingham, MA, USA) recipients for both bridge-to-transplant (BTT) and destination therapy (DT). Our primary objective was to determine the magnitude and durability of reverse remodeling seen with CF pumps, as assessed by the decrease in left ventricular end diastolic dimensions (LVEDD) and degree of mitral regurgitation (MR) when comparing pre-LVAD to post-LVAD parameters.
Single-institutional results with reverse remodeling using continuous glow
LVAD therapy
One hundred patients with advanced heart failure underwent implantation of a continuous flow LVAD at our institution from March 2006 to July 2011. This included 93 HM II LVADs and seven HVADs. Patients were implanted as BTT in 68 cases and as DT in 32 cases. Clinical records of these patients, including echocardiograms and right heart catheterizations, were retrospectively reviewed to determine how LVEDD and degree of MR were affected by CF-LVAD support at one and six months post-LVAD implantation. The procedures followed were in accordance with institutional guidelines and this review was performed with IRB approval.
Device management
Device speed was clinically adjusted to optimize flow, peripheral perfusion, organ function and LV decompression. Patients underwent periodic echocardiograms to evaluate the degree of LV decompression, aortic ejection, residual mitral regurgitation, position of the interventricular septum, RV function and severity of TR.
All patients were postoperatively anticoagulated on aspirin 81 mg daily as well as warfarin with a target INR of 1.8-2.5. Heart failure medications typically included a beta blocker, ACE inhibitor and diuretics, as well as sildenafil if there was significant residual pulmonary hypertension.
Echocardiography
Preoperative echocardiograms were reviewed and compared to echocardiograms at 1 and 6 months postoperatively. All echocardiograms were performed by staff cardiologists at Henry Ford Hospital. LVEDD, and the severity of MR based on a graded system was recorded for each patient. A graded qualitative system for RV function, as well as severity of TR was used for each patient. Additionally, RVEF and RVEDD were recorded. Finally, echocardiograms were reviewed for tricuspid annular plane systolic excursion (TAPSE) measurements preoperatively, at one and six months post-LVAD.
Hemodynamic variables
Hemodynamic variables were analyzed pre-LVAD implantation and compared to one and six months post-LVAD implantation values. These variables included CVP, PAP, pulmonary capillary wedge pressure (PCWP) and cardiac index (CI). Perioperative RV failure was defined as the need for intravenous inotropes for greater than 14 days postoperatively or a right ventricular assist device (RVAD).
Statistical analysis
Statistical analysis was conducted by a statistician from the Department of Biostatistics at Henry Ford Hospital. Data were represented as frequency distributions and percentages. Values of continuous variables were expressed as a mean ± standard deviation (SD). Continuous variables were compared using independent samples t-tests. Categorical variables were compared by means of Chi-square tests. For all analyses, a P value of <0.05 was considered statistically significant. Kaplan-Meier analysis was used to calculate survival along with a log-rank P value when comparing groups. Actuarial survival at one, three and five years post-implant were calculated by constructing life tables. All data were analyzed using SPSS 11.5 (SPSS Inc., Chicago, Illinois, USA).
Results
Demographics
There were 73 male and 27 female LVAD patients with a mean age of 52.8±11.9 years. Etiology of heart failure was coronary artery disease (ischemic cardiomyopathy) in 34 patients and non-ischaemic dilated cardiomyopathy in 66 patients. Median LVAD support time was 378.3 days, 371.5 days for BTT patients and 422.2 days for DT patients. Additional preoperative clinical characteristics of the patients are listed in Table 1.
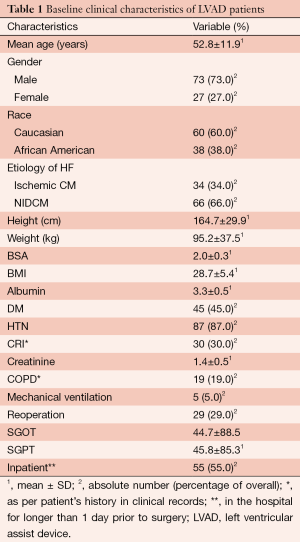
Full table
Echocardiographic data
LVEDD significantly decreased at one month post-LVAD implantation from 71.6±12.4 to 58.3±13.8 mm (P<0.001). The severity of MR was moderate or severe in 76.0% of patients preoperatively. At one month, only 8.0% had moderate or severe MR (P<0.001) and this was maintained at six months post-LVAD implantation (Table 2).
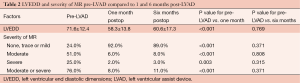
Full table
Improvement in right ventricular function and right-sided hemodynamics
From March 2006 to June 2012, 130 patients with chronic heart failure underwent implantation of a CF-LVAD at our institution. This included 122 HM II LVADs and eight HVADs. Patients were implanted as BTT in 76 cases and as DT in 54 cases. Patients with preoperative long-term LVADs (n=4) as well as patients who underwent concomitant tricuspid valve repairs during their LVAD implant (n=21) were not included in the analysis. Clinical records of the remaining 105 patients, including right heart catheterizations and echocardiograms, were retrospectively reviewed to determine how RV size and function were affected by CF-LVAD support at one and six months post-LVAD implantation.
Hemodynamic data
At one month post-LVAD implantation, CVP decreased from 12.4±5.9 to 8.7±4.5 mmHg (P<0.001), systolic PAP decreased from 52.3±14.1 to 36.8±11.3 mmHg (P<0.001) and PCWP decreased from 23.0±9.4 to 12.9±8.0 mmHg (P<0.001). Additionally, CI increased from 1.8±0.5 to 2.4±0.5 L/min/m2 (P<0.001) and RVSWI improved from 408.6±144.6 to 614.4±196.2 mmHg mL/m2 (P<0.001) (Table 3).
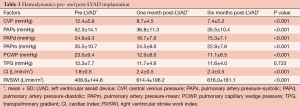
Full table
Echocardiographic data
Right ventricular function
RVEF increased from (33.1±4.9)% preoperatively to (40.4±6.2)% (P<0.001) at 1 month postoperatively, with a reduction in RVEDD from 36 to 31 mm (P=0.020). Qualitatively, RV function was moderately or severely reduced in 57.1% of patients preoperatively. At 1 month, only 33.3% had moderately or severely reduced RV function, and this improvement was maintained at 6 months post-LVAD implantation (P=0.008) (Table 4).
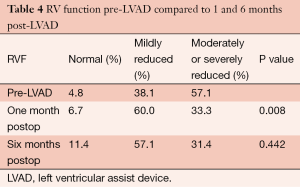
Full table
Qualitative assessment of severity of tricuspid regurgitation
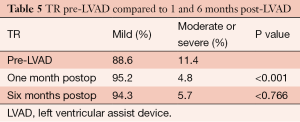
The severity of TR was moderate or severe in 11.4% of patients preoperatively. At 1 month, only 4.8% had moderate or severe TR and this improvement was also maintained at 6 months post-LVAD implantation (P<0.001) (Table 5).
Full table
Tricuspid annular plane systolic excursion (TAPSE)
TAPSE measurements were recorded for 92 (87.6%) patients. Mean TAPSE for patients was 1.1±0.4 cm preoperatively. This increased significantly to 1.9±0.4 cm at 1 month (P=0.004) and was maintained at 2.0±0.5 cm at 6 months post-LVAD implantation.
Right ventricular failure
Postoperative RV failure occurred in 11 patients. This included six patients who were treated with milrinone for an extended period of time and five patients who underwent RVAD placement. Among the six patients who required prolonged milrinone therapy, three were INTERMACS category IA preoperatively on inotropic support and an intra-aortic balloon pump. Milrinone was weaned off in five patients at 17 days, 18 days, 3 months in two patients and 4.5 months. One patient was transplanted on milrinone at 9 months. Among the five patients who required an RVAD, two were INTERMACS category IA preoperatively.
Discussion
Despite significant improvements in pump design and better short- and long-term outcomes with continuous flow pumps, LVAD therapy remains confined to very advanced, end-stage heart failure patients with severe functional limitations. Extension of this therapy to patients at earlier stages of disease is dependent on continued improvement in long-term outcomes as well as the potential for significant myocardial recovery with the option for device explantation. Left ventricular reverse remodeling is crucial to the concept of myocardial recovery. The clinical and pathologic sequelae of LV remodeling in heart failure are well defined and various options for intervention have been explored to slow or reverse the progression of the remodeling process. Passive constraint devices including the Acorn CorCap and the Myosplint have had experimental success in inducing LV reverse remodeling in heart failure patients but have not led to significant improvements in clinical outcomes (11,12). LVAD therapy, on the other hand, has significantly improved survival and quality of life in end-stage heart failure patients. However, there is little data concerning LV reverse remodeling with continuous flow device therapy. The ultimate goal of LV recovery, namely device explantation, remains a rare clinical event. Understanding LV reverse remodeling is an important precursor in being able to promote this recovery process.
Reductions in CVP and PAP with concomitant RV unloading are important goals of LVAD therapy because sustained RV function is crucial to good long-term outcomes. Our patients manifested considerable unloading of the RV, as demonstrated by significant reductions in CVP and PAP at 1 and 6 months post-implantation. There is the potential for these devices to induce RV dysfunction by causing leftward shifting of the interventricular septum (13). Our practice is therefore to obtain serial echocardiograms during the initial days and weeks of support to determine the LVAD speed and ventricular loading conditions that will maximally decompress the LV without excessively shifting the interventricular septum and exacerbating RV dysfunction. These studies are performed at rest, although there may be additional benefits to performing them during activity as well. Device speed is increased until there is optimal decompression of the LV, with no bowing of the interventricular septum and either no residual or trivial MR. Additionally, we aim to have the aortic valve open several times per minute.
In conclusion, our results demonstrated that CF-LVAD support induced immediate and sustained reverse remodeling of the LV, as measured by significant reductions in LVEDD and severity of MR. Our data are consistent with similar studies with PF pumps and confirm the findings of smaller series with CF pumps (11-13). Given the substantial and consistent reduction in the severity of MR after LVAD implantation, we do not believe that concomitant mitral valve repair is necessary during the initial operation. To date, we have not performed any concomitant mitral valve procedures on patients undergoing LVAD implantation. Residual MR can generally be reduced by increasing the RPM’s, which yields greater LV decompression. For the rare patient with symptomatic, refractory or severe residual MR, mitral valve repair may be warranted.
The engineering paradigm of CF will likely be the platform for future LVAD device development because of the associated advantages of CF pumps, namely improved survival, quality of life and decreased pump-related adverse events. While we have observed LV recovery leading to prolonged device explantation in only one of these patients, a more detailed examination of the reverse remodeling process, such as analysis of myocyte size, regression of fibrosis, cellular hypertrophy, collagen content, gene expression and various biomarkers of recovery, such as cardiac tumor necrosis factor-alpha, will extend our understanding of the remodeling process beyond just volume unloading of the LV and may allow for more focused attempts at long-term LV recovery. Additionally, it is possible that partial unloading of the LV may induce substantial reverse remodeling and promote myocardial recovery, although this hypothesis requires further preclinical and clinical analysis.
Study limitations
This is a retrospective review and limitations include potential inaccuracy of data retrieved from medical records. Additionally, the number of patients in this study was relatively small, thus limiting the statistical power of the analysis and conclusions. Further studies with more patients and longer follow-up will be useful.
Acknowledgements
Funding: This project was provided by Henry Ford Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008;86:1227-34; discussion 1234-5. [PubMed]
- Xydas S, Rosen RS, Ng C, et al. Mechanical unloading leads to echocardiographic, electrocardiographic, neurohormonal, and histologic recovery. J Heart Lung Transplant 2006;25:7-15. [PubMed]
- Klotz S, Jan Danser AH, Burkhoff D. Impact of left ventricular assist device (LVAD) support on the cardiac reverse remodeling process. Prog Biophys Mol Biol 2008;97:479-96. [PubMed]
- Wohlschlaeger J, Schmitz KJ, Schmid C, et al. Reverse remodeling following insertion of left ventricular assist devices (LVAD): a review of the morphological and molecular changes. Cardiovasc Res 2005;68:376-86. [PubMed]
- Vatta M, Stetson SJ, Jimenez S, et al. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J Am Coll Cardiol 2004;43:811-7. [PubMed]
- Thompson LO, Skrabal CA, Loebe M, et al. Plasma neurohormone levels correlate with left ventricular functional and morphological improvement in LVAD patients. J Surg Res 2005;123:25-32. [PubMed]
- Mann DL, Kubo SH, Sabbah HN, et al. Beneficial effects of the CorCap cardiac support device: five-year results from the Acorn Trial. J Thorac Cardiovasc Surg 2012;143:1036-42. [PubMed]
- Schenk S, Reichenspurner H, Groezner JG, et al. Myosplint implantation and ventricular shape change in patients with dilative cardiomyopathy- first clinical experience. J Heart Lung Transplant 2001;20:217. [PubMed]
- Chow E, Farrar DJ. Right heart function during prosthetic left ventricular assistance in a porcine model of congestive heart failure. J Thorac Cardiovasc Surg 1992;104:569-78. [PubMed]





