Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: the Brigham and Women’s Hospital experience
Introduction
Minimally invasive aortic valve replacement (mini AVR) was first described in 1993 (1), and subsequently popularized in 1996 and 1997 as an alternative to conventional full sternotomy (FS) for patients with isolated pathology of the aortic valve and ascending aorta without coronary artery disease (2-4). A variety of techniques have been described, including parasternal (2,3), infra-axillary (5), lower hemi-sternotomy (HS) (6) and transverse sternotomy (7) approaches. Today, mini AVR is performed primarily via upper HS or right anterior thoracotomy incisions (8).
Favorable results have led mini AVR to become a standard procedure in many high volume centers (9). In addition to the smaller incision and improved cosmesis, numerous studies report a reduction in post-operative bleeding, transfusion requirements, rates of atrial fibrillation, length of mechanical ventilation, length of intensive care unit (ICU) and hospital stay, as well as post-operative pain with no difference in mortality (3,10-17). However, these benefits have been inconsistent and widespread acceptance of the procedure has been elusive. Critics point to longer cardiopulmonary bypass and aortic cross-clamp times (8,16), which in general tend to predict worse outcomes in cardiac surgery, though this is not consistently seen for mini AVR (10,18,19). The minimally invasive approach limits the ability to control left ventricular distention, and some surgeons do not use it for severe aortic insufficiency. Finally, the correlation between surgical volume and outcomes is well established (20). Because minimally invasive surgery requires a new skill-set, the associated learning curve is a deterrent for many surgeons.
In this paper, we compare the outcomes of mini AVR via upper HS and conventional AVR via FS at our institution since 2002.
Methods
Patient selection
The Institutional Review Board at The Brigham and Women’s Hospital (BWH) approved this study. Since 2002, 1,319 patients underwent isolated mini AVR via HS. Re-operations with previous valve and coronary artery bypass graft interventions were included; prior aortic operations were excluded. Alternative approaches, including mini-thoracotomy and paramedian sternotomy techniques comprised a minority of operations (<20 patients) and were excluded for comparison analysis. The decision to perform HS was at the discretion of the operating surgeon in concert with patient preference. During the same time frame, 1,702 patients meeting these same criteria underwent FS. Propensity matching techniques (see below) were used to derive a control cohort from this patient pool.
Patient characteristics, laboratory and operative data, and in-hospital outcomes were recorded at the time of patient presentation and extracted from hospital electronic medical records. Data were coded to the Society of Thoracic Surgeon’s Adult Cardiac Database, version 2.52 specifications, unless otherwise noted. Long-term mortality and reoperation were collected by routine patient follow-up, query of the Social Security Death Index, and/or our internal long-term follow-up data repository.
Operative technique
We have previously published our preferred technique for mini AVR (20,21). We use an upper HS through a 6 to 9 cm skin incision (Figure 1A) (22). The sternum is transected horizontally at the level of the 4th intercostal space, taking care to avoid injury to the right internal mammary artery. Thymol fat is dissected and pericardial sutures are placed and retracted to the dermis while the sternal retractor is temporarily removed, thereby exposing the aorta and operative field. Hemodynamic changes at this point may develop from distortion of the inferior vena cava and can be mitigated by loosening the right-sided stay sutures to re-establish right heart pre-load. The patient is fully heparinized and the ascending aorta assessed for a safe cannulation site with an epiaortic ultrasound.

We use a 7 mm Terumo Sarns Soft Flow-Flow extended aortic cannula (Terumo, Tokyo, Japan). For venous drainage, a three stage Medtronic 29 Fr MC2X venous cannula has been used for right atrial drainage, but most were cannulated in the right femoral vein with a percutaneous Medtronic Biomedicus femoral 21 Fr venous cannula (Medtronic, Minneapolis, MN, USA) and positioned under transesophageal echocardiographic guidance. Cardiopulmonary bypass is initiated and the aorta is directly cross-clamped. Antegrade cardioplegia is given and, if greater than mild aortic insufficiency is present, supplemented by direct ostial delivery after the aortotomy. Though not routinely used, retrograde cardioplegia can be given via cannulation of the right atrium or through the right internal jugular vein. Figure 1B illustrates typical operative exposure through upper HS (22).
Reoperative mini AVR is accomplished by establishing peripheral cardiopulmonary bypass (axillary or femoral artery and femoral vein cannulation) prior to performing the HS. We use a minimal dissection technique to expose the aorta. Prior left internal mammary arterial grafts are left patent and the heart is arrested with systemic hyperkalemia; the operation proceeds under moderate to deep hypothermia (20-24 °C). The safety of this approach has been previously reported (21).
Statistical analysis
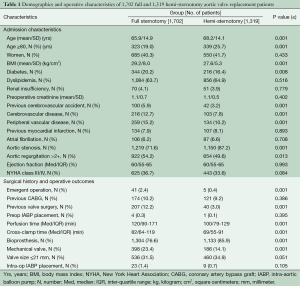
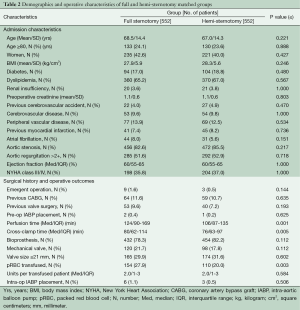
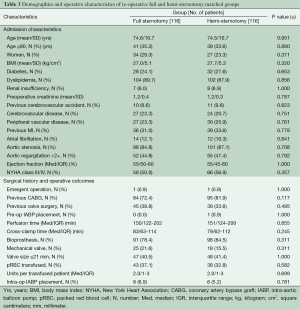
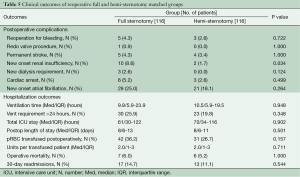
Because the surgical approach was not random, propensity-score matching techniques were used to create matched case (HS) and control (FS) cohorts in order to reduce selection bias. Propensity-score matching was done using a multivariable logistic regression model that was built in two steps. Potential predictors of minimally invasive surgery selection were identified based on univariate analyses of differences between groups (Table 1), literature review, known confounding covariates for the outcomes of interest, and clinical judgment. These variables were categorized as patient characteristics or related to treatment decisions, and separate forward stepwise logistic regressions were run for each variable set, including evaluating interaction effects. Any variable significant at a P≤0.15 was entered into the final enter-method logistic regression model, including an interaction variable between the surgeon and the year of surgery to control for variability in case mix and surgical practice over time. The resulting adjusted predicted value for each patient was used to conduct the propensity matching. Our model resulted in 552 matched pairs between FS and HS; baseline characteristics of matched patients are presented in Table 2. A second, similar matching process was performed from the entire cohort to further compare re-operative full and hemi sternotomy patients, resulting in 116 matched pairs (Table 3).
Full table
Full table
Full table
Short-term outcomes of interest included intensive care unit (ICU) and hospital length of stay, transfusion requirements, and operative mortality. Mid and long-term survival were calculated as well as freedom from aortic valve re-intervention due to any cause.
Univariate analyses of normally distributed continuous variables were conducted using a t-test with Levine’s test of homogeneity of variance; analyses of non-parametric variables were done using Mann-Whitney U tests. Continuous variables are presented as means ± standard deviation or medians (Med) and inter-quartile ranges (IQR) as appropriate. Survival and time to re-intervention were evaluated using Kaplan-Meier analyses. The criterion for significance was P≤0.05. Statistics were performed using SPSS 13.0 (Chicago, IL, USA).
Results
HS vs. FS matched cohorts
As can be seen in Table 1, at baseline there were many significant and clinically important differences between the FS and HS cohorts, prompting the decision to use propensity matching methodology. The characteristics of the resulting matched cohorts of 552 HS and 552 FS patients are presented in Table 2. Mean age in HS was 67±14.3 years compared with 68.5±14.4 years in FS (P≤0.221). The two groups demonstrated similar distributions in percent octogenarians, gender, and body mass index (BMI). Aortic stenosis was the most common indication for surgery in both groups: 85.5% in HS vs. 82.6% in FS, P≤0.217. Baseline ejection fractions were similar: median 60% (IQR 55-65); distribution of percent New York Heart Association class III or IV was 37% in HS vs. 35.8% in FS, P≤1.0. A trend towards higher emergent operations was seen in FS, though this difference did not meet statistical significance: HS 0.5% (3/552) vs. FS 1.6% (9/552), P≤0.144.
Median cardiopulmonary bypass and cross clamp times were shorter in the HS group: 106 minutes (IQR 87-135) vs. 124 minutes (IQR 90-169), P≤0.001, and 76 minutes (IQR 63-97) vs. 80 minutes (IQR 62-114), P≤0.005, respectively. The groups were similar in respect to valve type and size of prostheses implanted (Table 2). Intra-operatively, patients undergoing FS were more likely to get packed red blood cell transfusion, 27.9% vs. 20.0%, P≤0.003, although there were no differences between groups in the amount per transfused patient: the median in both groups was two units (IQR 1-3) P≤0.584.
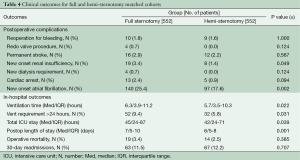
Additional in hospital outcomes are summarized in Table 4. HS patients had less time on the ventilator (median 5.7 h, IQR 3.5-10.3 vs. 6.3 h, IQR 3.9-11.2, P≤0.022), shorter intensive care unit stay (median 42 h, IQR 24-71 vs. 45 h, IQR 24-87, P≤0.039), and shorter hospital stay (median 6 days, IQR 5-8 vs. 7 days, IQR 5-10, P≤0.001) compared with FS. Other short term clinical outcomes were similar, except a decreased incidence of new onset atrial fibrillation was observed in HS: 17.6% (97/522) vs. 25.4% (140/522), P≤0.002. No difference was seen in operative mortality: HS 2.5% (14/522) vs. FS 3.4% (19/522), P≤0.385.
Full table
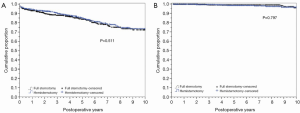
Long-term survival was comparable in HS and FS patients (Figure 2A). Figure 2B shows time to aortic valve re-intervention for any cause, demonstrating no difference between valves placed via hemi sternotomy or FS approaches.
Reoperative HS vs. FS matched cohorts
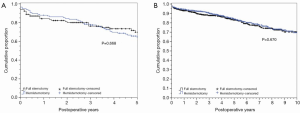
Table 3 outlines the demographics and operative characteristics of the 116 matched pairs of reoperative sternotomy patients. Reoperative FS and reoperative HS groups were similar in baseline characteristics. No differences were seen in procedure characteristics including similar median cardiopulmonary bypass and perfusion times for re-operative FS versus reoperative HS: 150 minutes, IQR 122-202 vs. 151 minutes, IQR 124-200, P≤0.855, and 83 minutes, IQR 63-114 vs. 79 minutes, IQR 62-112, P≤0.245, respectively. Valve size and prosthesis type were also similar, as was the intraoperative incidence of transfusions in both groups. In hospital outcomes were not different for reoperative FS and reoperative HS patients (Table 5), including similar median ICU and hospital lengths of stay: 61 minutes, IQR 30-122 vs. 70 minutes, IQR 34-116, P≤0.902, and 8 days, IQR 6-13 vs. 8 days, IQR 6-11, P≤0.501, respectively. The difference in post-operative new onset atrial fibrillation seen in the overall matched cohort was not seen in the reoperative matched groups. Reoperative HS and FS patients also had no difference in survival or time to aortic valve re-intervention for any cause (Figure 3A,B).
Full table
Discussion
Although the benefits of mini AVR surgery have been widely reported, widespread adoption has not occurred and some skeptics are calling for additional evidence (8,16). We present a robust, propensity-matched comparison of aortic valve replacement via HS and FS approaches from our institution since 2002. The major findings in our study are that patients undergoing isolated AVR via HS had less time on the ventilator, shorter ICU and hospital length of stays with comparable short and long-term survival compared to the FS group. These findings corroborate other reports demonstrating similar in-hospital benefits of mini AVR (10,13,16). Numerous studies have shown that these improved in-hospital outcomes likely result from decreased post-operative pain, facilitating quicker return of pulmonary function and mobilization (3,14-16).
Another major advantage promoted by champions of minimally invasive surgery is the decreased transfusion requirements compared with FS. In our study, we found a decreased rate of transfusion in the HS group. These results are consistent with prior meta-analyses (16), as well as another recent propensity-matched study by Gilmanov and colleagues, which reported half as many units transfused per patient in the minimally invasive AVR group compared to FS patients (10). Interestingly, Gilmanov and colleagues reported no difference in reoperations for bleeding, a finding consistent with our own results (Table 4). Along with increased transfusion requirements, blood loss in the first 24 h after surgery is higher with FS (13,16,23,24).
The simple principle that less dissection yields less chance for bleeding and therefore minimizes transfusion requirements may also extend to reoperative surgery. In a report of our early experience with reoperative partial upper HS, we showed decreased operative and post-operative transfusions, as well as less chest tube drainage in the first 24 h (23). Our current investigation did not corroborate these initial transfusion findings, consistent with our recent report that octogenarians undergoing reoperative AVR have no difference in bleeding and transfusion requirements (25). Detailed data on chest tube drainage was not available.
Another important difference seen in the HS group was the decreased incidence of new onset atrial fibrillation during the post-operative period (HS: 17.6% vs. FS: 25.4%). While the etiology of atrial fibrillation remains complex and multi-factorial (26), similar findings have been reported (10,27), though pooled data remains inconclusive (16). Differences in new onset atrial fibrillation have also been observed with other minimally invasive approaches such as the right anterior minithoractomy (18,28). Recent studies comparing ministernotomy and right minithoracotomy for AVR also show possible advantages to the minithoracotomy, including less transfusion and hospital length of stay (18). Additional studies and data pooled from multiple institutions are needed to confirm these findings.
The HS affords the surgeon a “familiar exposure” and does not require special instruments. This may minimize the learning curve compared to a mini-thoracotomy approach and may ultimately facilitate faster cardiopulmonary bypass and aortic cross clamp times as reported in our cohort. Brown and colleagues performed a meta-analysis of 26 studies and concluded that differences in operative times were not significant, but many of these studies reported data from early surgical experiences with minimally invasive sternotomy prior to 2002 (16). An important advantage of the HS is the option for conversion to conventional, FS. We have previously published data on the conversion rate from our first 10 years of mini AVR experience, reporting an incidence of 4%, with poor exposure being the most common reason for conversion (29). Pooled data now estimate a conversion rate of 3% (95% confidence interval 1.8-4%) (16).
Our long term clinical outcomes confirm the safety and efficacy of valves placed through a HS approach. No differences were seen in overall survival or time to aortic valve re-intervention for any cause (Figures 2,3).
Study limitations
This study represents a retrospective, single center experience and is therefore subject to all limitations inherent to such a design. While the propensity matching technique used has been well validated, a limitation of the methodology is that it cannot address bias introduced by unmeasured variables, thus it is possible our results reflect the influence of unknown factors other than the effect of the treatment on the treated. However, this design does allow us to evaluate a patient sample that is representative of the isolated AVR population at our institution, and our sample size was robust. We chose to begin our study period in 2002 for two reasons: (I) this is approximately 5 years after the commencement of routine mini AVRs and, therefore, represents a period after the learning curve; and (II) completeness of data available in our electronic records. While this may be a limitation in the sense that we excluded patients undergoing mini AVR, it represents a truer comparison of surgical expertise in the two techniques.
Conclusions
To our knowledge, this represents the largest propensity matched analysis comparing HS and FS for aortic valve replacement to date. In addition to the intrinsic cosmetic advantages, we have validated the essential clinical benefits of this minimally invasive technique, including decreased transfusion requirements, ventilation times, ICU and hospital length of stay without compromising short and long-term survival compared with conventional AVR via FS. The decreased incidence of atrial fibrillation seen in the HS group is a promising finding supported by other reports in the literature but requires further investigation. While HS should be compared with alternative minimally invasive techniques, it remains an excellent option with favorable outcomes that should be considered part of the routine armamentarium of cardiac surgeons in the modern era.
Acknowledgements
Authors’ contribution: RN performed data collection, drafted and edited manuscript. MB contributed to data collection and initial draft. IG edited operative technique section and final draft editing. TK contributed to discussion points and final draft editing. SM performed data analysis including propensity modeling. ML contributed to data interpretation and final editing. LC developed study design and final editing.
Disclosure: The authors declare no conflict of interest.
References
- Rao PN, Kumar AS. Aortic valve replacement through right thoracotomy. Tex Heart Inst J 1993;20:307-8. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Benetti FJ, Mariani MA, Rizzardi JL, et al. Minimally invasive aortic valve replacement. J Thorac Cardiovasc Surg 1997;113:806-7. [PubMed]
- Ito T, Maekawa A, Hoshino S, et al. Right infraaxillary thoracotomy for minimally invasive aortic valve replacement. Ann Thorac Surg 2013;96:715-7. [PubMed]
- von Segesser LK, Westaby S, Pomar J, et al. Less invasive aortic valve surgery: rationale and technique. Eur J Cardiothorac Surg 1999;15:781-5. [PubMed]
- Moreno-Cabral RJ. Mini-T sternotomy for cardiac operations. J Thorac Cardiovasc Surg 1997;113:810-1. [PubMed]
- Malaisrie SC, Barnhart GR, Farivar RS, et al. Current era minimally invasive aortic valve replacement: techniques and practice. J Thorac Cardiovasc Surg 2014;147:6-14. [PubMed]
- ElBardissi AW, Shekar P, Couper GS, et al. Minimally invasive aortic valve replacement in octogenarian, high-risk, transcatheter aortic valve implantation candidates. J Thorac Cardiovasc Surg 2011;141:328-35. [PubMed]
- Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013;96:837-43. [PubMed]
- Dogan S, Dzemali O, Wimmer-Greinecker G, et al. Minimally invasive versus conventional aortic valve replacement: a prospective randomized trial. J Heart Valve Dis 2003;12:76-80. [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive aortic valve surgery in the elderly: a case-control study. Circulation 2003;108 Suppl 1:II43-7. [PubMed]
- Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006;81:1599-604. [PubMed]
- Bonacchi M, Prifti E, Giunti G, et al. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73:460-5; discussion 465-6. [PubMed]
- Candaele S, Herijgers P, Demeyere R, et al. Chest pain after partial upper versus complete sternotomy for aortic valve surgery. Acta Cardiol 2003;58:17-21. [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-9. [PubMed]
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534. [PubMed]
- Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg 2013;145:1222-6. [PubMed]
- Dewey TM, Herbert MA, Ryan WH, et al. Influence of surgeon volume on outcomes with aortic valve replacement. Ann Thorac Surg 2012;93:1107-12; discussion 1112-3. [PubMed]
- Gosev I, Kaneko T, McGurk S, et al. A 16-year experience in minimally invasive aortic valve replacement: context for the changing management of aortic valve disease. Innovations (Phila) 2014;9:104-10; discussion 110.
- Tabata M, Khalpey Z, Shekar PS, et al. Reoperative minimal access aortic valve surgery: minimal mediastinal dissection and minimal injury risk. J Thorac Cardiovasc Surg 2008;136:1564-8. [PubMed]
- Shekar PS. Minimal access aortic valve surgery through an upper hemisternotomy approach. Operative Techniques in Thoracic and Cardiovascular Surgery: A Comparative Atlas 2010;15:321-35.
- Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [PubMed]
- Liu J, Sidiropoulos A, Konertz W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. Eur J Cardiothorac Surg 1999;16 Suppl 2:S80-3. [PubMed]
- Kaneko T, Loberman D, Gosev I, et al. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2014;147:155-62. [PubMed]
- Hogue CW Jr, Hyder ML. Atrial fibrillation after cardiac operation: risks, mechanisms, and treatment. Ann Thorac Surg 2000;69:300-6. [PubMed]
- Mächler HE, Bergmann P, Anelli-Monti M, et al. Minimally invasive versus conventional aortic valve operations: a prospective study in 120 patients. Ann Thorac Surg 1999;67:1001-5. [PubMed]
- Miceli A, Murzi M, Gilmanov D, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg 2014;148:133-7. [PubMed]
- Tabata M, Umakanthan R, Khalpey Z, et al. Conversion to full sternotomy during minimal-access cardiac surgery: reasons and results during a 9.5-year experience. J Thorac Cardiovasc Surg 2007;134:165-9. [PubMed]





