Results of open thoracoabdominal aortic aneurysm repair
Background: Open surgical repair of thoracoabdominal aortic aneurysms (TAAAs) enables the effective replacement of the diseased aortic segment and reliably prevents aneurysm rupture. However, these operations also carry substantial risk of perioperative morbidity and mortality, principally caused by the associated ischemic insult involving the spinal cord, kidneys, and other abdominal viscera. Here, we describe the early outcomes of a contemporary series of open TAAA repairs.
Methods: We reviewed the outcomes of 823 open TAAA repairs performed between January 2005 and May 2012. Of these, 209 (25.4%) were Crawford extent I repairs, 264 (32.1%) were extent II, 157 (19.1%) were extent III, and 193 (23.5%) were extent IV. Aortic dissection was present in 350 (42.5%) cases, and aneurysm rupture was present in 37 (4.5%). Adjuncts used during the procedures included cerebrospinal fluid drainage in 639 (77.6%) cases, left heart bypass in 430 (52.2%), and cold renal perfusion in 674 (81.9%).
Results: The composite endpoint, adverse outcome—defined as operative death, renal failure that necessitated dialysis at discharge, stroke, or permanent paraplegia or paraparesis—occurred after 131 (15.9%) procedures. There were 69 (8.4%) operative deaths. Permanent paraplegia or paraparesis occurred after 42 (5.1%) cases, stroke occurred after 27 (3.3%), and renal failure necessitating permanent dialysis occurred after 45 (5.5%).
Conclusions: Although open surgical repair of the thoracoabdominal aorta can be life-saving to patients at risk for fatal aneurysm rupture, these operations remain challenging and are associated with substantial risk of early death and major complications. Additional improvements are needed to further reduce the risks associated with TAAA repair, particularly as increasing numbers of patients with advanced age and multiple or severe comorbidities present for treatment.
Key words: Aorta, abdominal; aorta, thoracic; aortic aneurysm, thoracic/surgery; cardiovascular surgical procedures
Introduction
Without surgical treatment, patients with thoracoabdominal aortic aneurysms (TAAAs) have dismal survival (1). Consequently, the development of surgical techniques that enabled surgical repair of the thoracoabdominal aorta represented a major advance in the field of cardiovascular surgery. Since the first iterations of open TAAA repair, these operations have made possible the effective replacement of the diseased aortic segment and have reliably prevented aneurysm rupture. However, TAAA repair has also carried substantial risk of perioperative morbidity and mortality, principally caused by the associated ischemic insult involving the spinal cord, kidneys, and other abdominal viscera. Advances in perioperative care, surgical technique, and adjuncts for organ protection have led to excellent outcomes after open TAAA repair in the current era. Nevertheless, surgical teams still face substantial challenges in further reducing the risks of associated complications, especially as they treat an increasingly aged patient population with limited physiologic reserve due to comorbid diseases. Here, we describe the early outcomes of a contemporary series of open TAAA repairs.
Patients and methods
Patient population
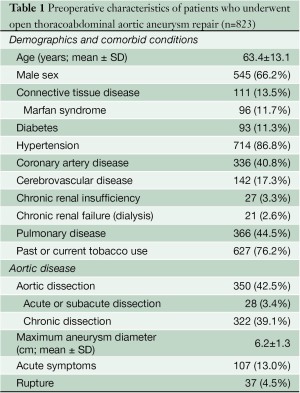
Under a protocol approved by the institutional review board at Baylor College of Medicine, we identified 844 consecutive patients who underwent TAAA graft replacement operations between January 17, 2005, and May 31, 2012. Of these, 819 (97%) provided written informed consent for data analysis. These 819 patients underwent a total of 823 TAAA graft replacement operations and thus compose the study group (Table 1). Perioperative data from January 2005 through May 2006 were collected retrospectively through review of all available medical records (155 repairs, 18.8%), and data from June 2006 through May 2012 were collected prospectively during inpatient rounds and concomitant review of medical records (668 repairs, 81.2%). Data related to 509 of the 823 procedures (61.8%) have been reported in a recent publication (2).
Full table
Study variables
Regarding preoperative variables, renal failure was defined as the need for dialysis, whereas renal insufficiency was defined as a serum creatinine level greater than 3.0 mg/dL (in patients not receiving dialysis). In patients with aortic dissection, dissection was considered acute when surgery was performed within 14 days of onset, subacute when surgery was performed 15-60 days after onset, and chronic when surgery was performed more than 60 days after onset. The maximum TAAA diameter was based on measurements of all segments of the descending thoracic aorta and the abdominal aorta; the largest of these measurements was used as the maximum diameter.
The extent of aortic repair was classified according to the system described by Crawford (Figure 1) (3). Extent I repairs were defined as repairs that involved most or all of the descending thoracic aorta and the upper abdominal aorta but did not involve the infrarenal aorta. Extent II repairs involved most or all of the descending thoracic aorta and extended into the infrarenal abdominal aorta. Extent III repairs involved the distal half, or less, of the descending thoracic aorta (beginning below the 6th rib) and involved varying portions of the abdominal aorta. Extent IV repairs involved most or all of the abdominal aorta and began at the diaphragm. Of the 823 TAAA repairs, 209 (25.4%) were extent I, 264 (32.1%) were extent II, 157 (19.1%) were extent III, and 193 (23.5%) were extent IV.

Operative mortality (early death) was defined as death before discharge or within 30 days of surgery. For the purposes of calculating operative mortality and overall length of stay, patients were considered to have been discharged if they were transferred to rehabilitation centers or skilled nursing facilities, but not if they were transferred to long-term care or other acute-care facilities. To determine early vital status after discharge, clinical and research staff telephoned patients, patients’ family members, and patients’ physicians. If no telephone contact was made, letters were sent to the patient’s last known address. When direct contact was not possible, the Social Security Death Index was used to determine vital status. All length-of-stay calculations excluded patients who died before discharge.
Postoperative acute renal dysfunction was defined as an increase in the serum creatinine level to twice its baseline value or the need for hemodialysis during the postoperative period. Acute renal failure was defined as the need for either transient hemodialysis (temporary dialysis that was discontinued before discharge) or permanent hemodialysis (dialysis at discharge or at time of death). Permanent spinal cord deficits were defined as those present at hospital discharge and were further classified according to their severity — paraplegia (inability to move legs) versus paraparesis (leg weakness) — and the timing of onset—immediate versus delayed (4). Cardiac complications included atrial and ventricular arrhythmia, cardiac failure (including inotropic support longer than 48 hours, placement of an intraaortic balloon pump, or both), myocardial infarction, permanent pacemaker placement, and pericardial effusion requiring drainage. Pulmonary complications included acute respiratory distress syndrome, atelectasis necessitating bronchoscopy, pleural effusion requiring drainage, pneumonia, and respiratory failure necessitating ventilator support for more than 48 hours and/ or tracheostomy. When the incidence of complications was calculated, multiple events (e.g., arrhythmia and pericardial effusion) within a category (e.g., cardiac complications) were treated as one occurrence. To better assess the overall incidence of major complications, we defined the composite endpoint, adverse outcome, as the occurrence of any of the following: operative death, renal failure that necessitated permanent dialysis, stroke, and permanent paraplegia or paraparesis (2,5).
Data are presented as n (%) for all discrete variables. Continuous variables are presented as mean ± standard deviation or median and interquartile range, as appropriate.
Surgical techniques
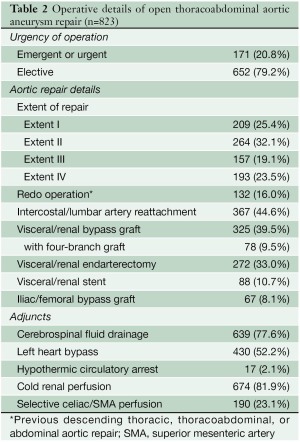
Our current techniques for performing graft replacement of the thoracoabdominal aorta are described in detail elsewhere in this issue of Annals of Cardiothoracic Surgery (6,7) and in other recent publications (8-14). Details of the techniques used in the current series are presented in Table 2. We routinely employed a multimodal approach to organ protection (7,9,14); the specific adjuncts used varied according to the planned extent of aortic replacement and various other clinical factors. In all extents, we employed moderate heparinization (1 mg/kg) and mild passive hypothermia (with a target nasopharyngeal temperature near 33 ℃). When we had access to the renal artery ostia, we administered intermittent cold renal perfusion to prevent acute renal failure (8,15); in most cases, we used cold crystalloid solution as the perfusate, although a few patients received cold blood renal perfusion during a clinical trial (16). When possible, we also selectively reimplanted intercostal or lumbar arteries and used sequential cross-clamping. When performing extent I or II TAAA repairs, we used cerebrospinal fluid (CSF) drainage and left heart bypass (LHB), and we often perfused the celiac axis and the superior mesenteric artery (SMA) with isothermic blood from the LHB circuit (9,14,17,18). We did not routinely use evoked potential monitoring. We only used cardiopulmonary bypass and hypothermic circulatory arrest if it was not possible to apply a proximal aortic clamp.
Full table
Results
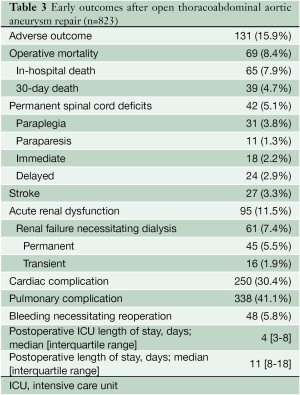
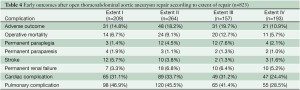
Postoperative outcomes for the overall group and for the different extents of repair are presented in Tables 3 and 4, respectively. The composite endpoint, adverse outcome, occurred after 15.9% of procedures, and the overall operative mortality rate was 8.4%; the risks of adverse outcome and death were both highest in the patients who underwent extent III repairs. The most common complications were those involving the pulmonary system. Permanent paraplegia or paraparesis occurred after 5.1% of repairs; deficits had immediate onset in 43% of cases and had delayed onset in 57%. The incidence of permanent paraplegia was highest after extent III repair. Acute renal dysfunction occurred after 11.5% of the operations; 64% of patients with renal complications received dialysis. Of those needing postoperative dialysis, 74% remained on dialysis at the time of discharge. The incidence of permanent renal failure was highest after extent II repairs. Stroke occurred after 3.3% of cases; 81% of the strokes occurred after extent I or II repairs.
Full table
Full table
Discussion
Although open surgical repair of the thoracoabdominal aorta can be life-saving to patients at risk for fatal aneurysm rupture, these operations remain challenging and are associated with substantial risk of early death and major complications. In this report of our contemporary experience with TAAA repairs, we found that 15.9% of procedures resulted in a major adverse outcome, including the 8.4% that resulted in early mortality. Our recent analysis of risk factors associated with complications after TAAA repair revealed that an increased risk of operative mortality was associated with increasing age, pulmonary disease, cerebrovascular disease, aneurysm without dissection, acute presentations (i.e., increasing clamp and ischemic times, and the need for hypothermic circulatory arrest) (2). Independent predictors of adverse outcome included increasing age, pulmonary disease, cerebrovascular disease, and aneurysm rupture; extent IV TAAA repair was associated with a lower risk of adverse outcome. These findings have significant implications because the general population is aging and has increasingly significant comorbidity; surgeons are evaluating increasing numbers of patients whose age and comorbidities place them at substantial risk for complications.
Summarizing the literature regarding the contemporary results of TAAA repair is complicated by inherent differences in approaches to reporting. For example, many reports combine data from descending thoracic aortic aneurysm repairs with data from TAAA repairs. Others exclude extent IV TAAA repairs (19) or focus on a specific clinical subgroup of interest [e.g., patients less than 60 years old (20)]. Furthermore, authors often use different definitions when reporting neurologic and renal complications. Despite these limitations, it is clear that experienced centers are currently achieving respectable early survival and complication rates after open TAAA repair. Recently reported outcome rates range from 3.4% to 8.9% for 30-day mortality, 3.4% to 12.3% for in-hospital mortality, 1.5% to 5.8% for paraplegia, 3.7% to 6.3% for stroke, and 1.7% to 14.3% for renal failure (19-27). It is noteworthy that the different series exhibit substantial variation in important clinical factors (19-27).
For example, the proportion of patients who underwent extent II TAAA repairs ranges between 8.7% and 47.6%. In addition, recent reports show persistent and considerable variability in the surgical approaches and adjuncts that are used at different centers: the use of hypothermic circulatory arrest, renal perfusion, and intercostal artery reattachment ranges between none and routine, and the use of CSF drainage ranges between 75% and 100%. Furthermore, some reports focus on recent experience with contemporary treatment strategies (20-23), whereas others describe 10 or more years of surgical experience (19,24-27). The more comprehensive series provide excellent opportunities for evaluating trends in patient management and results, as well as for assessing long-term outcomes, but these reports are likely to be confounded by changes in treatment approach that occurred during the study period. The more contemporary series tend to comprise patients treated with similar approaches and are particularly useful as comparison groups when new alternative techniques are assessed.
Despite the limitations in reporting and the variation in treatment approaches among the various recent single-center series, these studies support the concept that specialized, high-volume centers are able to achieve respectable outcomes after these formidable operations. In contrast, the few existing population studies of national or statewide data for TAAA repair tend to show much worse outcomes than single-center studies do. For example, a statewide, nonfederal analysis of 1,010 patients revealed an early mortality rate of 25% (28), and national analyses show mortality rates of 20% to 22% (29,30). This difference in results may be at least partially explained by the inclusion of low-volume centers in these multicenter studies. In the statewide analysis, 40% of the patients were treated at centers that averaged only 1 TAAA repair per year (28), and in the national analysis, 37% of patients were treated in low-volume centers (i.e., facilities that performed a median of 1 TAAA repair per year) (30).
In conclusion, contemporary approaches to open TAAA repair yield respectable early results. Nevertheless, these operations continue to carry a substantial risk of early adverse outcomes. Additional improvements are needed to further reduce the risks associated with TAAA repair, particularly as surgeons are faced with increasing numbers of patients with advanced age and comorbidities.
Acknowledgements
The authors thank Laurie Fondren, Michael Hughes, Jennifer Parenti, RN, and Jeffrey Whorton, RHIA, for managing subject enrollment and data collection, Scott A. Weldon, MA, CMI, for creating the illustration, and Stephen N. Palmer, PhD, ELS for providing editorial support.
Disclosure: The authors declare no conflict of interest.
References
- Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg 1986;3:578-82.
- Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79; discussion 579-81.
- Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404.
- Wong DR, Coselli JS, Amerman K, et al. Delayed spinal cord deficits after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2007;83:1345-55; discussion 1355.
- LeMaire SA, Miller CC 3rd, Conklin LD, et al. A new predictive model for adverse outcomes after elective thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2001;71:1233-8.
- Coselli JS, LeMaire SA, Weldon SA. Extent II repair of thoracoabdominal aortic aneurysm secondary to chronic dissection. Ann Cardiothorac Surg 2012;1:394-7.
- de la Cruz KI, LeMaire SA, Weldon SA, et al. Thoracoabdominal aortic aneurysm repair with a branched graft. Ann Cardiothorac Surg 2012;1:381-93.
- Bhamidipati CM, Coselli JS, LeMaire SA. Perfusion techniques for renal protection during thoracoabdominal aortic surgery. J Extra Corpor Technol 2012;44:P31-7.
- Coselli JS, LeMaire SA. Tips for successful outcomes for descending thoracic and thoracoabdominal aortic aneurysm procedures. Semin Vasc Surg 2008;21:13-20.
- Coselli JS, LeMaire SA. Surgical technique for extent I, II, and III thoraco-abdominal aortic aneurysms. In: Chiesa R, Melissano G, Zangrillo A, et al, eds. Thoraco-Abdominal Aorta: Surgical and Anesthetic Management. Milan, Italy: Springer Milan, 2011:327-43.
- Huh J, LeMaire SA, Coselli JS. Descending and thoracoabdominal aortic aneurysms. In: Cohn LH, ed. Cardiac Surgery in the Adult. 4th ed. New York: McGraw-Hill Professional, 2012:1081-104.
- Tsai P, LeMaire SA, Coselli JS. Thoracoabdominal aneurysms. In: Sellke FW, Ruel M, eds. Atlas of Cardiac Surgical Techniques. Philadelphia, PA: Saunders/Elsevier,2010:310-25.
- Tsai PI, LeMaire SA, Coselli JS. Management of descending thoracic and thoracoabdominal aortic aneurysms. In: Cameron JL, eds. Current Surgical Therapy. 10th ed. Philadelpha, PA: Elsevier Saunders, 2011:717-27.
- Vaughn SB, LeMaire SA, Collard CD. Case scenario: anesthetic considerations for thoracoabdominal aortic aneurysm repair. Anesthesiology 2011;115:1093-102.
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8.
- LeMaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19.
- Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1999;67:1931-4; discussion 1953-8.
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
- Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2011;141:953-60.
- Di Luozzo G, Geisbüsch S, Lin HM, et al. Open repair of descending and thoracoabdominal aortic aneurysms and dissections in patients aged younger than 60 years: superior to endovascular repair? Ann Thorac Surg 2012. [Epub ahead of print].
- Bisdas T, Redwan A, Wilhelmi M, et al. Less-invasive perfusion techniques may improve outcome in thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2010;140:1319-24.
- Keyhani K, Miller CC 3rd, Estrera AL, et al. Analysis of motor and somatosensory evoked potentials during thoracic and thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:36-41.
- Lima B, Nowicki ER, Blackstone EH, et al. Spinal cord protective strategies during descending and thoracoabdominal aortic aneurysm repair in the modern era: the role of intrathecal papaverine. J Thorac Cardiovasc Surg 2012;143:945-952.
- Schepens MA, Heijmen RH, Ranschaert W, et al. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5.
- Zoli S, Roder F, Etz CD, et al. Predicting the risk of paraplegia after thoracic and thoracoabdominal aneurysm repair. Ann Thorac Surg 2010;90:1237-44; discussion 1245.
- Conrad MF, Ergul EA, Patel VI, et al. Evolution of operative strategies in open thoracoabdominal aneurysm repair. J Vasc Surg 2011;53:1195-201.
- Patel VI, Ergul E, Conrad MF, et al. Continued favorable results with open surgical repair of type IV thoracoabdominal aortic aneurysms. J Vasc Surg 2011;53:1492-8.
- Rigberg DA, McGory ML, Zingmond DS, et al. Thirtyday mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg 2006;43:217-22; discussion 223.
- Derrow AE, Seeger JM, Dame DA, et al. The outcome in the United States after thoracoabdominal aortic aneurysm repair, renal artery bypass, and mesenteric revascularization. J Vasc Surg 2001;34:54-61.
- Cowan JA Jr, Dimick JB, Henke PK, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg 2003;37:1169-74.





