Total endoscopic sutureless aortic valve replacement: rationale, development, perspectives
Introduction
The balance between transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) is definitely evolving towards the choice of the transcatheter option for high-risk patients (1). Indeed, there will soon be debate regarding the technique of choice for intermediate-risk patients and focus on the pivotal question “will we still have to ablate the underlying pathology?”. The value of aortic tissue ablation still appears to be fundamental, and the answer remains strictly surgical until safe transcatheter decalcificators become available. The issue is addressed for several reasons: calcium still acts as a generator of paravalvular leakages (PVL) and major adverse cardiovascular events (MACE) during TAVI, relative to calcium scores and distribution (2). Despite encouraging preliminary improvements reported with new generation TAVI valves (3,4), the most serious concern for transcatheter-based therapy is related to post-procedural neurological injury. In fact, the incidence of apparent stroke occurs twice as frequently with TAVI than with SAVR. TAVI does not remove valvular tissues and hence may result in persistence of a valvular source of embolization. When compressed against the aortic wall, this may potentially act as a nidus for calcified emboli, followed by potential deterioration of neurocognitive dysfunction and quality of life (5).
SAVR is continually evolving with minimal access procedures becoming more widespread thanks to the latest turning point: sutureless technology. Beyond the advantages linked to the elimination of sutures and knots (6,7), some of those devices carrying a Nitinol frame offer the possibility of being compressed, enabling their passage through a thoracoscopic trocar.
Total endoscopic aortic valve replacement (TEAVR) aims to further reduce surgical wall chest trauma by avoiding sternal fractures or costal spreading. Clinical advantages have already been shown during atrial septal defect (ASD) closure surgery (8), thus favoring total endoscopic approach over ministernotomy and minithoracotomy.
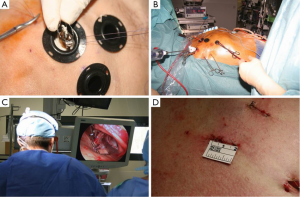
Before proceeding to a clinical experience of TEAVR, proof of concept was achieved in cadavers (Figure 1) with the use of a five trocars setting (two working ports, a camera trocar, and two trocars for the management of a pulmonary vent) as previously described (9). The real challenge was to define a step-by-step sequence to deliver the Medtronic 3f Enable bioprosthesic sutureless valve under exclusive control of a camera. From November 2012 to April 2013 thirteen patients received a 3f Enable valve through a video-assisted mini-thoracotomy technique, consisting of keeping the valve compressed with a Prolene stitch in the cuff and cutting it when the valve was visualized with the camera at the proper level for expansion (Figure 2). In our protocol, this delivery technique (10), if successful after a feasibility phase, would be transferred in a close chest setting. The results of the first 13 patients treated through video-assisted procedure were satisfactory, with median cross-clamping time of 64 (range, 37-120) minutes, CPB time of 92 (range, 69-125) minutes and absence of PVL or block.
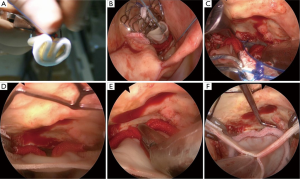
In this initial phase of TEAVR, patients were strongly selected based firstly on anatomical requirements. Suitable patients should have a sufficiently long intrapericardial ascending aorta in order to keep a comfortable distance between the aortic clamp, the cardioplegia needle, and the aortotomy. Consequently safe placement of the aortic closing sutures is facilitated. We selected patients with a central line of the ascending aorta longer than 5 cm. A second criterion of selection was the presence of a sufficient peri-aortic working space (>2 cm between the inferior margin of the sternum and the aortic anterior wall) to enable the management of the minimally invasive needle holder and grasper during the ascending aorta running suture. Finally, the central axis of the proximal aortic root, together with the operative axis of the third intercostal space, should not create an angle >45° at the center of the aortic valve. Not abiding to this criterion would result in an excessive traction of instruments over margins of the aortotomy. Secondly, patient selection was dependent upon preoperative ventricular ejection fraction to be superior to 45% because of the longer duration of clamping time required. Finally, hemodynamic iliac and femoral artery stenoses have to be excluded.
Between June and December 2013, five patients were selected according to the previous criteria for a TEAVR Enable implantation. All procedures were technically successful; nevertheless one conversion to second space minithoracotomy was necessary after positioning of the valve. In this particular patient, the aortotomy was performed too proximally in order to achieve a safe endoscopic closure within a reasonable cross-clamping time; the minithoracotomy was performed with an incision connecting the second space working trocar and the optics trocar, in order to close the aorta under direct view. The overall median clamping and CPB time were 116 (range, 77-124) and 164 (range, 116-181) minutes, respectively. All patients could be extubated within 24 hours. Intraoperative and post-operative echography controls excluded presence of PVL. No pacemaker implantations were required, and all patients left the unit at postoperative day seven.
TEAVR seems to be safe but reserved to selected patients according to anatomical criteria and tolerance for longer operative durations. With respect to operative times, the technique must be improved to become reproducible. Catherine Otto, Co-Chair of the writing committee for the new ACC/AHA valve guidelines recently stated that if larger studies would show that this approach to aortic valve replacement is effective with an acceptable mortality and morbidity, it would offer an intermediate option between standard SAVR and TAVI (11).
Perspectives
The evolution of minimally invasive aortic valve surgery is linked to the development of technologies such as sutureless valves, especially in severe aortic stenosis pathology. Compared to minimally invasive mitral valve surgery, the possibility of performing a thoracoscopic procedure with a cross clamp time comparable with conventional surgery is more likely because of the different nature of the two pathologies. Most mitral valves are surgically repaired, with variable outcomes and a reconstruction that can be complex and require sutures, while aortic valve stenosis requires a more constant action: ablation of tissues, calibration, and valve implantation. The nature of the latter is more suitable for a potential “device-dependent”, rather than operator dependent, procedure that can be performed reliably. It relies on the development of sizers, endoscopic decalcification instruments, second- generation sutureless valves and aortic closure devices in order to target a 4-step, quick, minimally traumatic treatment of the stenosed aortic valve.
Endoscopic sizers
Aortic intraoperative sizing is limited for endoscopic surgery using existing sizers. The existing sizers are rigid and incompressible, with only small diameter sizers (19 or 21 mm) that can enter the cutaneous orifice of 20 mm of the main thoracoscopic trocar without requiring skin enlargement, and without conflict with the margin of the ribs. Secondly, the holder of an ideal sizer should be malleable to facilitate a perfect alignment with the central line of the aortic root when the position of the trocars in not perfectly axed.
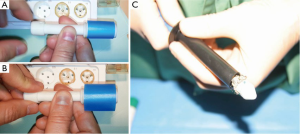
From a theoretical standpoint, sizing could be exclusively image based as described in the initial experience. However, the annulus elasticity should be considered in sizing for a sutureless valve implant, irrespective of the surgical access. The diameter of the sutureless valve should ideally be selected using a sizer that can provide an immediate feedback force to the operator to help assess the adequate “internal diameter/resistance to radial expansion” ratio and thereby select the most appropriate size. Also, such a sizer should be progressive in order to cover the measurement of intermediate sizes of the prosthesis (20, 22, 24, 26 mm) that are not always provided by industry calibrators. We have recently developed and patented a prototype, however this is yet to be used in clinical practice (Figure 3A,B).
Decalcificators
A dedicated instrument for decalcification is not currently available; nor are minimally invasive aortic instruments. The ideal endoscopic decalcification instrument should enable rapid and safe valve decalcification without embolization and avoid repeated insertion and extraction of the device through the aortic root.
Second-generation sutureless valves
First-generation sutureless valves have been conceived for open surgery and not for TEAVR. We have evaluated the ratio of compression of the 3f Enable and Perceval valves: the 3f Enable valve is available in sizes from 19 to 27 mm requires a 20 mm trocar to be inserted in the thorax; only the small size of the Perceval model fits a 15 mm trocar (Figure 3C). A 20 mm trocar is necessary for the other sizes. The Edwards Intuity model (Edwards, Irvine, CA, USA) is composed by a Magna incompressible bioprosthesis and does not fit trocars.
Second generation sutureless valves should ideally allow the use of lower diameter trocars. The mechanism of delivery should be easy and intuitive for TEAVR approach. Use of percutaneous valves could be considered with this objective. However, concerns have been raised regarding the lack of grip once the native calcium is ablated and the potential lesions that the radial force of those valves could cause on a decalcified annulus.
In our initial TEAVR experience, we selected the 3f Enable valve because of the previous experience with this valve in our department. We are now proceeding to cadaver TEAVR with the balloon expandable Sorin Perceval (Sorin Biomedica Cardio Srl, Saluggia, Italy) model, which seems to have a shorter learning curve. However, adjustments in case of malposition appear less possible.
Aortic closure device
Aortic closure devices are also needed, as aortic closure remains a challenge in endoscopic procedures. The EWAC1000 suturing device (Edwards, Irvine, CA, USA) was tested on cadavers, without a time advantage over conventional suturing. A learning curve effect cannot be excluded. The articulated arm seems to be too long to easily move in a reduced working space compared with left atrium closure during minimally invasive mitral surgery. Robotic technology appears promising in speeding aortic closure, however this approach has not yet been tested in our anatomy laboratory.
Conclusions
The totally endoscopic aortic valve replacement has proven to be technically feasible with the current technology in selected patients. However, it is far from being a mature technique as indicated by operative times. Potential evolution towards a reproducible, quick and safe procedure relies on the development of specifically adjuvant devices.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Leber AW, Kasel M, Ischinger T, et al. Aortic valve calcium score as a predictor for outcome after TAVI using the CoreValve revalving system. Int J Cardiol 2013;166:652-7. [PubMed]
- Seiffert M, Conradi L, Kloth B, et al. Single-centre experience with next-generation devices for transapical aortic valve implantation. Eur J Cardiothorac Surg 2015;47:39-45. [PubMed]
- Taramasso M, Pozzoli A, Latib A, et al. New devices for TAVI: technologies and initial clinical experiences. Nat Rev Cardiol 2014;11:157-67. [PubMed]
- Fanning JP, Wesley AJ, Platts DG, et al. The silent and apparent neurological injury in transcatheter aortic valve implantation study (SANITY): concept, design and rationale. BMC Cardiovasc Disord 2014;14:45. [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: Early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [PubMed]
- Santarpino G, Pfeiffer S, Concistrè G, et al. Perceval S aortic valve implantation in mini-invasive surgery: the simple sutureless solution. Interact Cardiovasc Thorac Surg 2012;15:357-60. [PubMed]
- Morgan JA, Peacock JC, Kohmoto T, et al. Robotic techniques improve quality of life in patients undergoing atrial septal defect repair. Ann Thorac Surg 2004;77:1328-33. [PubMed]
- Vola M, Fuzellier JF, Chavent B, et al. First human totally endoscopic aortic valve replacement: an early report. J Thorac Cardiovasc Surg 2014;147:1091-3. [PubMed]
- Vola M, Campisi S, Anselmi A, et al. Video-assisted minithoracotomy approach: technical developments towards totally endoscopic sutureless aortic valve replacement. J Card Surg 2014;29:494-6. [PubMed]
- Husten H. French Surgeons Perform First Aortic Valve Surgery Without Opening The Chest. Forbes, March. Web, 2014.





