Staged approach for spinal cord protection in hybrid thoracoabdominal aortic aneurysm repair
Introduction
At present, there are three available options for surgical thoracoabdominal aortic aneurysm (TAAA) repair: conventional open surgery, total endovascular repair, and a hybrid approach, involving conventional surgical and endovascular techniques. Each modality has a different risk/benefit profile, requiring careful patient selection. Spinal cord injury (SCI) due to compromised blood supply following extensive segmental artery (SA) sacrifice is a daunting complication observed with all three approaches.
A thorough understanding of the response of the spinal cord vasculature to SA sacrifice is essential to minimize the incidence of SCI after open or endovascular repair of TAAA. For over a decade, perioperative spinal cord protection during TAAA repair has been investigated in Dr. Griepp’s laboratory at The Mount Sinai Hospital in New York. Clinical and experimental work have provided insight into the anatomy of the extensive vascular network surrounding the spinal cord, and its dynamic response to SA sacrifice. This new understanding of spinal cord perfusion has been termed the Collateral Network Concept (CNC) (1).
The staged approach to TAA repair
The staged approach emerged from the clinical observation that patients undergoing aneurysm repair in two stages suffered less SCI than patients with the same extent of SA sacrifice in one procedure (2). The anatomy of the collateral network was elucidated by studying casts of the vascular system in pigs. Subsequently, further studies demonstrated that SA occlusion leads to profound anatomic alterations within the collateral network perfusing the spinal cord, preserving blood flow by enlarging and adding to collateral pathways (3). In 2010, these findings fueled an experimental study in pigs by Zoli et al., who demonstrated that all SAs could be sacrificed in an open procedure without causing SCI if SA ligation is undertaken in a staged fashion (4).
The evolution of endovascular techniques - anticipating eventual total endovascular repair of TAAA - prompted a subsequent experiment to investigate the impact on spinal cord perfusion of extensive and immediate SA coverage by endovascular stent-graft deployment (5). In line with clinical hybrid repair, thoracic endovascular aortic repair (TEVAR) was used in place of surgical SA sacrifice via a left-sided thoracotomy. The results and their clinical implications are briefly presented.
Data summary
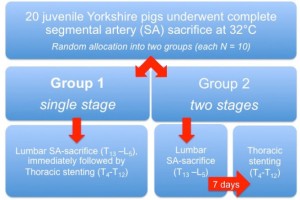
After randomization into two groups (each n=10), 20 juvenile Yorkshire pigs underwent open lumbar SA sacrifice in combination with endovascular coverage of all thoracic SAs (Figure 1). In Group 1, complete SA sacrifice (T4-L5) was achieved during one procedure. Group 2 pigs underwent extensive SA sacrifice in two stages, 7 days apart: TEVAR (stage 2, T4-T12) 1 week after open lumbar SA ligation (stage 1, T13-L5). The protocol was approved by the local institutional review board. Open abdominal SA sacrifice was undertaken as in clinical practice: under mild hypothermia (32 °C) in a serial fashion, with a 3-minute interval between adjacent SAs. Mean arterial pressure (MAP) and collateral network pressure (CNP) were directly monitored using tunneled catheters. For CNP measurements, the first lumbar SA (L1) was cannulated. As this location represents the anticipated center of SA sacrifice, it allows for precise monitoring of even minimal CNP changes. Postoperative hind limb function was evaluated by means of a modified Tarlov score, reaching from 0 (no voluntary movement) to 9 (full hind limb function). Animals with a score greater than 6 at the end of the 5-day protocol were considered to have recovered. CNP measurements and Tarlov scoring were carried out daily for 5 days. Additionally, histopathologic analysis of the spinal cord was performed in all animals at the end of the protocol.
In Group 1, only 5 of 10 pigs regained normal spinal cord function postoperatively. The other 5 animals showed clinical sequelae of SCI: paraplegia or paraparesis (median Tarlov score in Group 1 =6). In contrast, all animals in Group 2 quickly recovered from the first procedure, reaching behavioral scores >6 within 48 hours. After completion of SA sacrifice as a consequence of thoracic stent grafting, all animals regained full hind limb function (median Tarlov score in Group 2 =9; P=0.0031).
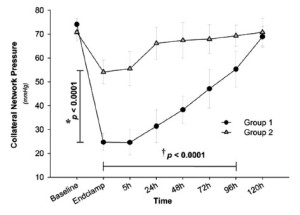
During the single stage procedure, CNP in Group 1 decreased to 34% of baseline [(74±2.4) to (25±3.6) mmHg; Figure 2]. During the 5 days of postoperative surveillance, CNP gradually recovered to its original value. In Group 2, CNP fell to only 55% of baseline after lumbar SA ligation [stage 1; (74±4.5) to (41±5.5) mmHg; P<0.0001]. After having returned to baseline within 5 days, subsequent TEVAR (stage 2) led to another significant but milder drop (P=0.002 vs. stage 1) from the restored CNP [(71±4.2) to (54±4.9) mmHg; Figure 2]. Histopathological analysis of harvested spinal cord tissue revealed significantly more ischemic alterations in Group 1 than in Group 2, notably in the lower thoracic (P=0.05) and lumbar regions (P=0.002).
Conclusions and clinical implications
The ultimate goal of the study was to enhance our understanding of how spinal cord perfusion is affected by endovascular repair: whether the response of the CNP differs when SA sacrifice is carried out suddenly rather than more gradually.
In the present model, SCI could be eliminated by dividing extensive SA sacrifice into two stages. Limited SA sacrifice during the first stage did not affect spinal cord function, but resulted in a vascular response which allowed CNP to recover to baseline levels within a couple of days. The anatomic reaction of the spinal cord vasculature to SA sacrifice has been meticulously studied in vascular cast models. Within a short time after SA sacrifice, profound changes occur in both the intraspinal and paraspinous vessels. After an early phase of vasodilatation, structural remodeling in terms of an increase in vessel diameter and density can be observed, stabilizing spinal cord blood supply (3). Thus, subsequent TEVAR led only to a mild drop in CNP, without occurrence of any clinically evident SCI (Figure 2). The results suggest that using a two-stage strategy allows hybrid TAAA repair to be undertaken with a much greater margin of safety with respect to SCI than using a single-stage approach (5).
Nevertheless, it is of note that, despite the uniformly excellent behavioral outcome, histopathologic analysis revealed ischemic spinal cord lesions in Group 2 animals. Although the histologic evidence of ischemia was not reflected by any impairment of function, these lesions suggest that established adjuncts of open aortic repair--such as hypothermia, cerebrospinal fluid drainage, and maintenance of high MAPs-- are likely to be important in hybrid and total endovascular approaches in order to ensure the safety of the spinal cord (5).
It has been suggested that sudden SA occlusion in endovascular repair may be even more dangerous for spinal cord viability than serial surgical ligation. Comparison of the CNP curves from the hybrid study with those from a comparable staged open repair study suggests a trend towards lower nadir pressures with thoracic stenting despite consistently higher MAPs in the hybrid series: there seems to be a difference in the pattern of CNP diminution and recovery between simultaneous occlusion of all SAs with TEVAR and sequential surgical ligation. During serial SA ligations, a brief drop and then gradual recovery of CNP is often noted between ligation of two neighboring SAs. This may be explained by immediate local vasodilatation to compensate for loss of SA input. The local response may not be as effective during TEVAR, where sudden occlusion of multiple SAs occurs, resulting in a steep drop of CNP. A comparison of the histological damage seen after a two-stage open surgical vs. hybrid protocol suggests that spinal cord injury is milder after more gradual open surgical SA sacrifice. The lower thoracic and the upper lumbar region may be at an increased risk for SCI in endovascular settings, in which local compensation is less effectively mobilized, especially if supporting subclavian or hypogastric artery inflow is reduced (5).
The critical question is how these experimental findings can be translated into clinical practice. Staged procedures inevitably expose the patient to a risk of rupture during the interval between the stages, requiring careful patient selection and surveillance. Furthermore, the specific morbidity and mortality risks of every additional procedure must be taken into account. Yet, given the results of the present study, and the fact that SCI is one of the most dreaded complications of TAAA repair, each patient’s suitability for staging should seriously be considered during preoperative planning of TAAA cases.
Acknowledgements
This research was supported by Grant No. 5R01HL45636 from the National Institutes of Health.
Disclosure: The authors declare no conflict of interest.
References
- Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg 2011;141:1020-8.
- Etz CD, Zoli S, Mueller CS, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2010;139:1464-72.
- Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice. J Thorac Cardiovasc Surg 2011;141:1029-36.
- Zoli S, Etz CD, Roder F, et al. Experimental two-stage simulated repair of extensive thoracoabdominal aneurysms reduces paraplegia risk. Ann Thorac Surg 2010;90:722-9.
- Bischoff MS, Scheumann J, Brenner RM, et al. Staged approach prevents spinal cord injury in hybrid surgicalendovascular thoracoabdominal aortic aneurysm repair: an experimental model. Ann Thorac Surg 2011;92:138-46; discussion 146.