Minimally invasive aortic valve replacement: the Leipzig experience
Introduction
Aortic stenosis is the most commonly acquired heart valve lesion in the Western world. It is usually caused by degenerative changes with complex calcification of the native leaflets and aortic annulus. Aortic valve replacement (AVR) has been the gold standard for treatment of severe aortic stenosis for the last 40 years. AVR was performed for decades via a median sternotomy with direct aortic and right atrial cannulation for cardiopulmonary bypass (CPB). The enthusiasm to perform minimally invasive cardiac surgery (MIC) emerged in the last decade of the twentieth century. A significant change in surgical techniques was heralded by the first MIC AVR performed by Cosgrove and Sabik in 1996 (1). MIC AVR has been reported to offer several benefits over conventional full sternotomy procedures such as better cosmesis, reduced pain, reduced surgical trauma, decreased blood loss, earlier functional recovery, and shorter hospital stay (2).
Various surgical approaches have been developed for MIC AVR surgery. Currently, the most commonly performed MIC access is via a partial upper sternotomy that extends into the third or fourth intercostal space, referred to as a ‘J’ or ‘L’-sternotomy or an inverted ‘T’-sternotomy (3,4). This approach is being commonly used at many centers around the world with excellent outcomes. However, a right anterior lateral mini-thoracotomy approach has been successfully employed in select centers (5). In the present series, the main focus is on our experience with minimally invasive AVR surgery over a partial upper sternotomy over the last decade.
Methods
We performed a database search in order to identify a total of 6,865 consecutive patients who underwent isolated AVR at our institution between January 2003 and March 2014. We did not exclude any patients based on surgical timing or aortic valve pathology. Patients requiring concomitant procedures such as coronary artery bypass grafting, mitral or other valve surgery, replacement of the ascending aorta, or atrial fibrillation ablation were excluded. Patients undergoing aortic valve repair were also excluded. In the final analysis, there were a total of 1,714 patients who underwent MIC AVR. Emergency conversion to sternotomy was required in four patients (0.3%) due to severe bleeding (two patients, 0.1%) or poor exposure of the aortic root (two patients, 0.1%), but these patients remained in the study for all subsequent comparisons in agreement with the “intention-to-treat” principle.
Patient selection
The decision of whether patients underwent a MIC AVR or a full sternotomy was predominantly made by the surgeon. Some surgeons exclusively used a MIC approach in virtually all patients. Other surgeons selectively applied MIC to those patients with a normal body-mass-index (BMI), a high risk of postoperative deep sternal wound infection, younger patients, or in those patients who explicitly requested a MIC approach. For a MIC approach, we do not perform any additional preoperative investigations such as CT scans, MRI or transesophageal echocardiography.
Surgical technique
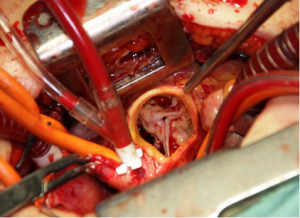
The ascending aorta and the aortic valve can be best accessed by opening the superior part of the sternum. The “inversed L” or “inversed T” shaped partial sternotomy is the current standard approach for minimally invasive aortic valve surgery, and involves a 5 cm midline skin incision performed downwards from about two fingers below the jugular notch. Usually the 3rd or 4th right/left intercostal space is carefully dissected lateral to the corpus of the sternum (Figure 1). The internal thoracic artery and vein usually remain intact. After partial sternotomy and opening of the pericardia, the pericardial rims are fixed to the incision using three to four retention stitches. This moves the whole heart, and especially the aorta with the aortic valve, anteriorly, providing a safe and effective access for valve repair or replacement. Cannulation for extracorporeal circulation is usually performed directly for minimally invasive aortic valve surgery. For the arterial cannula, the ascending aorta at its junction to the aortic arch, slightly above the pericardial fold, can be accessed quite easily. Routine aortic cannulas as from normal practice are used. Venous return is established by direct cannulation of the right atrial appendage. In some patients, the right atrial appendage is located immediately at the lower edge of the sternal incision; in others, it is located slightly deeper. Secured by a purse-string, the cannula may be positioned through the incision directly. However, caudal retraction of the cannula together with downward retraction of the right atrial appendage will improve exposure of the aortic root during aortic valve surgery. To accomplish this, the venous return cannula may be tunneled from the xiphoid region. After tunneling the cannula, good exposure of the aortic root will be achieved. At the end of the operation we use this incision to position the chest tube. When using this approach routinely, venous or arterial cannulation of the femoral vessels is rarely indicated. However, this could be performed when direct access is not possible. Myocardial protection consisted of antegrade or retrograde administration of blood cardioplegia with mild hypothermia, or antegrade administration of crystalloid cardioplegia (Bretschneider; Dr Franz Kohler, Chemie GmbH, Bensheim, Germany). A vent can be inserted via the right upper pulmonary vein or pulmonary artery to empty the left ventricle, decrease backflow of blood, and improve visualization of the aortic root. To prevent clinically significant gaseous emboli, carbon dioxide should be applied in all patients receiving minimally invasive valve surgery. Standard techniques were used to remove the native aortic valve and surrounding calcium, followed by standard insertion of a biological or mechanical prosthesis (Figure 2). De-airing is performed in a routine fashion after minimally invasive aortic valve surgery. The vent is stopped in time to allow the heart to fill spontaneously over some time while closing the aortotomy. Before aortic closure is completed, direct venting can be performed. In addition, a needle-vent is introduced into the aortic root. This allows further direct venting during the immediate period after releasing cross-clamp. Furthermore, continuous suction should be applied to the needle-vent for 5-10 minutes after opening the aorta. Conventional mobilization of the heart is hardly possible with the minimally invasive access since the heart cannot be mobilized through the small incision.
Transthoracic echocardiographic examinations were performed preoperatively, before discharge, and at every follow-up visit. Multi-plane transesophageal echocardiography was used intraoperatively or whenever additional information was required. Cardiac morphology and function as well as valve hemodynamics were assessed using standard measurements.
Follow-up
Follow-up was obtained by personal contact, mailed questionnaires, or by phone with patients and family members, with supplemental information being supplied by family physicians and referring cardiologists. Valve-related morbidity and mortality were evaluated according to standard guidelines (6). The mean follow-up interval was 4.7±4.3 years (range, 0-18 years) for a total of 7,971 patient years and was complete in 99.8%.
Statistics
Quantitative continuous variables are described with means ± standard deviation and quantitative discrete variables with absolutes and relatives frequencies throughout the manuscript. Early events (≤30 days post-implantation) were calculated as simple percentages. Kaplan-Meier actuarial analyses, including both early and late events, were performed with the Greenwood formula for variance. Multivariate Cox proportional hazards regression was performed to estimate the risk factors hazard ratios effects on mortality. All analyses were performed using SPSS version 21.0.
Results
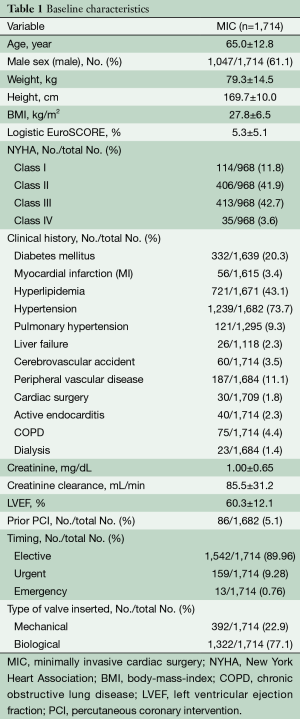
The mean age of the entire patient population was 65±12.8 years, of whom 25.6% (439/1,714) of these patients were older than 75 years, and 61.1% (1,047/1,714) were male. The preoperative patient characteristics and hemodynamic parameters are listed in Table 1. The anticipated risk for perioperative mortality according to the logistic EuroSCORE I was 5.3%±5.1%. Endocarditis was the indication for aortic valve surgery in 2.3% (39/1,714) of patients, and 25% (428/1,714) of these patients were obese with body mass indexes of over 30 kg/m2. Severe reduced ejection fraction (<30%) was seen in 49 patients (2.9%). Sixty-seven patients had prolonged cardiogenic shock, 10 patients (0.6%) were resuscitated and one patient needed extracorporeal membrane oxygenation therapy.
Full table
Examination of intraoperative variables revealed that MIC AVR patients had a mean cross-clamp time of 58.3±17.5 minutes and a CPB time of 82.9±26.7 minutes. The total length of surgery was 151.9±41 minutes. We implanted 392 mechanical and 1,322 biological aortic prostheses in our patients. Mean diameter of these aortic valve prosthesis was 23.5±2.0 mm.
Examination of early postoperative outcomes revealed that the rate of low cardiac output syndrome was 1.7% (29/1,714); intra-aortic balloon pump (IABP) implantation and extracorporeal membrane oxygenation (ECMO) implantation rates were 0.6% (10/1,714); transient neurologic deficit was 6.4% (109/1,714); and permanent neurologic deficit was 1.4% (24/1,714). Postoperative myocardial infarction (MI) with followed aortocoronary bypasses had an incidence of 0.4% (7/1,714), cardiac arrhythmias of mostly atrial fibrillation were seen in 34.3% (588/1,714), and acute kidney failure requiring dialysis occurred in 4.4% (75/1,714). The postoperative respiratory failure rate was 9.3% (159/1,714), and re-exploration for bleeding was 3.6% (62/1,714). Mean use of red blood cells was 2.1±4.2 packs, platelet concentrate was 0.2±1.1 packs, and fresh frozen plasma was 1.0±3.9 packs. Five patients (0.3%) had a deep wound infection after MIC AVR. Mean hospital stay was 12.4±7.4 days in our MIC AVR patients. Normally, our patients were discharged directly to three weeks of rehabilitation.
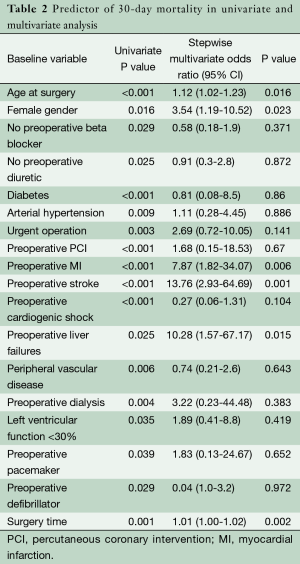
Univariate analysis for 30-day mortality of 48 preoperative and intraoperative variables revealed 19 risk factors (Table 2).
Full table
Significant variables in the univariate analysis were included in the multivariate analysis. In multivariate analysis, independent risk factors for hospital mortality were: age at surgery (P=0.016; OR, 1.1), length of surgery time (P=0.002; OR, 1.01), female gender (P=0.023; OR, 3.54), preoperative MI (P=0.006; OR, 7.87), preoperative stroke (P=0.001; OR, 13.76) and preoperative liver failure (P=0.015; OR, 10.28) (Table 2).
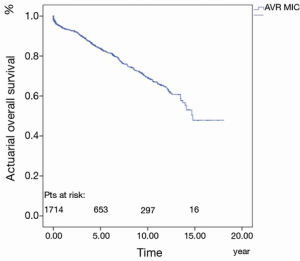
The 30-day, 1-, 5-, 10- and 15-year survival rate was 97.8%±0.4%, 94.0%±0.6%, 83.8%±1.1%, 69.4%±1.7% and 47.8%±4.7%, respectively (Figure 3).
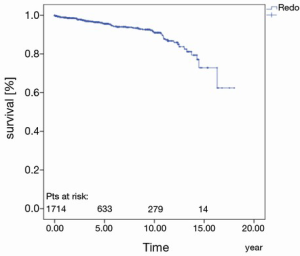
The prostheses-specific reoperation rate after five years, 10 and 15 years was 95.6%±0.7%, 91.0%±1.2% and 72.8%±5.6%, respectively (Figure 4).
Cox-regression analysis identified independent predictors for long-term mortality to be age over 75 years (P<0.001; OR, 3.5), preoperative dialysis (P<0.01; OR, 2.14), ejection fraction less than 30% (P=0.003; OR, 3.28) and urgent or emergency operation (P<0.001; OR, 2.3).
Discussion
Minimally invasive surgery represents a significant shift in the approach to traditional operative procedures in all surgical subspecialties. Since the first successful MIC AVR was performed by Cosgrove and Sabik in 1996 (1), MIC procedures have increased in number and evolved in technique. A number of previous publications have shown that MIC is superior to a conventional median sternotomy approach due to shorter hospitalization stay, reduced postoperative ventilation time, less blood loss, and lower transfusion rates (7-10). Although some studies have found contrary results with no obvious benefit for a minimally invasive approach (11,12), a meta-analysis has confirmed the above-mentioned advantages (2).
Although MIC AVR has several benefits, it is also associated with longer aortic cross-clamp, CPB and surgical times (2), probably because of the increased technical difficulty posed by the reduced surgical field. Sutureless and rapid deployment aortic valves have been recently developed in order to facilitate the performance of MIC surgery and thereby reduce operative times (13,14), but medium and long-term results with these devices remain unknown.
Glauber et al. recently performed a propensity-matched analysis comparing MIC to conventional AVR. In contrast to our study, these investigators used a right anterior mini-thoracotomy approach in all minimally-invasive surgical patients. They demonstrated a lower incidence of postoperative atrial fibrillation and blood transfusion, as well as shorter ventilation times and hospital stays in MIC patients with no difference in hospital mortality rates (5). Although these investigators prefer a right anterior mini-thoracotomy approach, most centers continue to perform MIC AVR surgery via an upper hemi-sternotomy.
Merk et al. compared early and long-term outcomes of MIC to conventional sternotomy in patients undergoing isolated bioprosthetic AVR. Patients undergoing mechanical AVR were excluded in order to minimize the effect of patient age on outcomes. A propensity matched analysis was performed in order to further limit differences in baseline risk factors between groups. After matching, there were no clinically significant differences in preoperative variables (15). This study had a significantly reduced in-hospital and long-term mortality rate in MIC AVR patients. MIC was associated with an absolute increase in postoperative survival of 7.5% and 4.9% at five and eight years respectively, when compared to conventional AVR surgery. Mihaljevic et al. noted a reduced mortality for patients undergoing MIC AVR (8). Glauber and colleagues also demonstrated excellent survival in MIS AVR patients three years postoperatively (96% vs. 88% for conventional AVR), but this difference did not reach statistical significance (5). In our study we have a 30-day survival rate at 97.8%±0.4%. At one year, our survival rate was 94.0%±0.6%. After five years, our survival rate was 83.8%±1.1%, and after 10 years, our survival rate was 69.4%±1.7%.
Multiple studies have reported longer myocardial ischemic times in MIC patients. However, this has not been shown to increase the rate of related adverse effects such as MI, IABP use, or low cardiac output syndrome in MIC patients (2,5,7,9,15). Jin and colleagues showed that a 20-minute-longer cross-clamp time in patient with stentless AVR versus patient with stented AVR (51 versus 72 minutes) had no effect on postoperative left ventricular function, morbidity, or mortality in a cohort of patients matched for age, gender, and valve size. There were no relevant differences between the two groups in overall hospital outcome. Intraoperative aortic cross-clamp time was longer in the stentless group, but the overall duration was acceptable because it did not result in any excess morbidity (16-18). In our study, there was no significant difference in cross-clamp time. It remains to be seen whether newer valve technologies, particularly sutureless or rapid deployment aortic valves, can reduce the myocardial ischemic times associated with MIC surgery.
We observed a low conversion rate to full sternotomy in MIC AVR patients (0.3%) in the current study, comparing favorably to other reports wherein this complication occurs in 2% to 2.6% of patients (9,19). We believe that detailed preoperative planning and a relatively large clinical experience may have contributed to our ability to avoid a full sternotomy in MIC patients.
Merk et al. report a lower incidence of postoperative delirium in patients undergoing full sternotomy AVR without any declaration. The reason for this lower incidence is not known, but may be related to technical difficulties in de-airing the left ventricle through a mini-sternotomy approach. One of the concerns about MIC AVR is the capability for de-airing the heart the end of the procedure. Although de-airing of the heart is more difficult than through a full sternotomy, we did not observe any increased clinical sequelae of air emboli in the patients undergoing MIC AVR. We routinely insufflate CO2 into the pericardium during all aortic valve procedures at our institution (15).
There are numerous reports of shorter hospitalization in MIC patients in the literature (2,5,7,19). In the German medical system, the impact on length of hospital stay may be explained by the vagaries of reimbursement in the hospital system, complicating comparisons of hospital stays to those from other countries.
Murtuza et al. found a markedly lower incidence of red blood cell transfusion in MIC patients versus patients with a full sternotomy in their meta-analysis (46.6% versus 63.5%, P<0.0001), as well as decreased postoperative blood loss 24 hours (2). In our study, we used an average of 2.1±4.2 red blood packs, 0.2±1.1 platelet concentrate packs and 1.0±3.9 fresh frozen plasma packs.
Our Cox multivariate logistic regression model identified independent risk factors for long-term mortality. The risk factor with the highest hazard ratio was age over 75 years, followed by reduced ejection fraction, urgent or emergency operation and preoperative dialysis. Multiple previous reports have documented that older age, reduced ejection fraction and preoperative dialysis negatively influences short- and long-term outcomes of patients undergoing aortic valve or any cardiac surgery (10,20-23).
Study limitations
The main limitation of our study is the fact that it is a retrospective, single center experience. The single center nature of our study may bring into question its generalizability, but it is important to note that a large number of surgeons performed the AVR procedures at our center and therefore the results should be generalizable to the cardiac surgery community. Finally, our analysis lacked information on postoperative quality of life during follow up.
Conclusions
MIC AVR through a partial sternotomy represents a shift in the approach to aortic valve surgery. MIC AVR is more technically demanding than conventional AVR and takes slightly longer to perform. We can conclude that minimal invasive AVR can be performed safely and effectively with very few perioperative complications. The early and long-term outcomes in these patients are acceptable. Once proficiency is acquired, the minimal access approach may be the procedure of choice for AVR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [PubMed]
- Svensson LG, D’Agostino RS. J” incision minimal-access valve operations. Ann Thorac Surg 1998;66:1110-2. [PubMed]
- Farhat F, Lu Z, Lefevre M, et al. Prospective comparison between total sternotomy and ministernotomy for aortic valve replacement. J Card Surg 2003;18:396-401; discussion 402-3. [PubMed]
- Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg 2013;145:1222-6. [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523-8. [PubMed]
- Bonacchi M, Prifti E, Giunti G, et al. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73:460-5; discussion 465-6. [PubMed]
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534. [PubMed]
- Tabata M, Umakanthan R, Cohn LH, et al. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur J Cardiothorac Surg 2008;33:537-41. [PubMed]
- Doll N, Borger MA, Hain J, et al. Minimal access aortic valve replacement: effects on morbidity and resource utilization. Ann Thorac Surg 2002;74:S1318-22. [PubMed]
- Christiansen S, Stypmann J, Tjan TD, et al. Minimally-invasive versus conventional aortic valve replacement--perioperative course and mid-term results. Eur J Cardiothorac Surg 1999;16:647-52. [PubMed]
- Detter C, Deuse T, Boehm DH, et al. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thorac Cardiovasc Surg 2002;50:337-41. [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [PubMed]
- Merk DR, Lehmann S, Holzhey DM, et al. Minimal invasive aortic valve replacement surgery is associated with improved survival: a propensity-matched comparison. Eur J Cardiothorac Surg 2014. [Epub ahead of print]. [PubMed]
- Jin XY, Westaby S. In vivo hemodynamic characteristics of porcine stentless aortic valves. Semin Thorac Cardiovasc Surg 2001;13:67-74. [PubMed]
- Jin XY, Gibson DG, Yacoub MH, et al. Perioperative assessment of aortic homograft, Toronto stentless valve, and stented valve in the aortic position. Ann Thorac Surg 1995;60:S395-401. [PubMed]
- Lehmann S, Walther T, Kempfert J, et al. Ten-year follow up after prospectively randomized evaluation of stentless versus conventional xenograft aortic valve replacement. J Heart Valve Dis 2011;20:681-7. [PubMed]
- Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006;81:1599-604. [PubMed]
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011;141:940-7. [PubMed]
- Lehmann S, Walther T, Leontjev S, et al. Mid-term results after Epic xenograft implantation for aortic, mitral, and double valve replacement. J Heart Valve Dis 2007;16:641-8; discussion 648. [PubMed]
- Leontyev S, Walther T, Borger MA, et al. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg 2009;87:1440-5. [PubMed]
- Leontyev S, Borger MA, Davierwala P, et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg 2011;91:1120-6. [PubMed]