Imaging and minimally invasive aortic valve replacement
Introduction
The percutaneous approach for transcatheter aortic valve replacement (TAVR) is becoming increasingly more common for high-risk patients, but the standard sternotomy approach is still used for aortic valve replacement (AVR) at many institutions. In our center, minimally invasive approaches (MIAVS) to the aortic valve are the preferred option for patients at reasonable risk for surgery. MIAVS provides safe and effective exposure for operations involving both the aortic valve and ascending aorta (1-4). Potential advantages over conventional median sternotomy include decreases in the length of hospital stay, hospital costs, pain, recovery time and requirement for packed red blood cells (PRBC) (5,6). Most commonly, the limited sternotomy extends from the sternal notch to the right 4th intercostal space (ICS). The feasibility of this approach relies on the proximity of the aortic valve to the 4th intercostal space (ICS). Variations in the location of the valve along the cranial-caudal or lateral planes can increase the complexity of the procedure.
The quality of the exposure is inversely proportional to both the length of the procedure and the likelihood of conversion to a full sternotomy. There are several alternatives to the standard upper-hemisternotomy, including extending the ‘J’ into the right 3rd or 5th ICS; using the inverted T partial sternotomy by extending into both right and left ICS, shifting to the lower hemi-sternotomy extending from the xiphoid to the right 3rd ICS; or sparing the sternum with a right paramedian transverse thoracotomy in the 2nd ICS. The success of any of these approaches depends on proper visualization optimized by preoperative planning (7). Preoperative imaging analysis allows for predictable assessment of the quality of the exposure before making the initial incision.
Preoperative imaging for standard aortic valve replacement (AVR)
Standard preoperative imaging for aortic valve surgery includes transthoracic echocardiography (TTE) and coronary catheterization for patients with any risk factors for coronary disease. This baseline imaging will reveal additional valve or coronary disease that requires intervention. In general, when patients require concomitant coronary artery bypass grafting, the combined operation is performed through a full median sternotomy. The 2014 American Heart Association/American College of Cardiology guidelines on valvular heart disease also state that “other ancillary testing such as transesophageal echocardiography (TEE), computed tomography (CT) or cardiac magnetic resonance imaging, stress testing and diagnostic hemodynamic cardiac catheterization may be required to determine the optimal treatment for a patient with valvular heart disease” (8).
A thorough history and physical examination will expose significant comorbidities that may affect the decision to perform a minimally invasive approach. In general, until an operator gains significant experience and confidence, he or she should be cautious about performing MIAVS in obese patients, older patients, or those with several comorbidities, in order to limit procedural and bypass duration and optimize cardiac protection. Patients with high society of thoracic surgeons (STS) risk scores (>8%) should be evaluated by a multidisciplinary heart team to determine if they are best served by TAVR.
Thin-sliced CT angiography of the chest, abdomen and pelvis as well as a TEE is routine components of the TAVR workup to assess annulus size, coronary origins, and access vessel characteristics. A chest CT is not typically ordered for a standard sternotomy AVR except on a few occasions: bicuspid aortic valve to rule out aneurysmal degeneration; reoperations to assess for safety of reentry; or when there is evidence of aortic calcification on other imaging such as chest X-ray or fluoroscopy. In most of these cases, a non-contrast CT will suffice unless one is interested in tracking the course of an arterial structure such as the left internal thoracic artery (LITA).
Preoperative imaging for minimally invasive aortic valve replacement (AVR)
Once the decision is made to undergo a minimally invasive approach, the surgeon has committed him/herself to a procedure with increased complexity and additional potential pitfalls. It is critical to understand the relationship of the valve to landmarks on the bony thorax, the presence of calcification on the ascending aorta and the status of groin vessels if needed for peripheral cannulation. If the patient’s glomerular filtration rate is within acceptable limits, a contrast enhanced thin slice CT scan with 3D reconstruction provides the greatest clarity of the mediastinal structures. If peripheral cannulation is anticipated, the CT can be extended through the entire aorta or a femoral arterial duplex may be obtained to elucidate the quality of the femoral vessels.
We typically use a multi-detector CT to acquire axial, sequential images with prospective gating after administration of a low-osmolar contrast agent. For optimization of anatomic evaluation, multi-planar reconstruction, maximum intensity projections, and advanced 3D off-line post-processing are performed on a dedicated stand-alone workstation (AcquariusNET, TeraRecon, Foster City, California, USA). In some patients, renal dysfunction limits their ability to tolerate the contrast. Although contrast enhanced studies provide finer detail, non-contrast CT images can also be reconstructed with reasonable detail.
Amar et al. published a small series of patients that had non-contrast CT with 3D reconstruction prior to mini AVR. The location of the aortic valve relative to the skin by CT was nearly identical to its relation to the skin in the operating room. This allowed them to plan the exact location and length of the mini incision (9). Glauber et al. routinely use non-contrast axial CT imaging prior to a right paramedian thoracotomy for AVR and demonstrated its utility in improving safety of the procedure (10).
CT imaging and 3D volumetric reconstruction software has evolved to the point of showing extremely fine details of the heart, soft tissue and bony thorax. We recently published a representation of our current practice using 3D reconstructed CT images to guide our operative approach (7). Figure 1 shows that the aortic valve is situated well below the 4th ICS. A standard upper hemi-sternotomy extending into the 4th ICS was predicted to provide excellent exposure which proved to be the case. Conversely, Figure 2 shows that the aortic valve is pushed far inferiorly by a long ascending aorta. This may have been too challenging to replace through a standard upper hemi-sternotomy. Using a lower hemi-sternotomy, extending from the xiphoid to the right 3rd ICS, we still offered our patient a minimally invasive approach without compromising exposure.
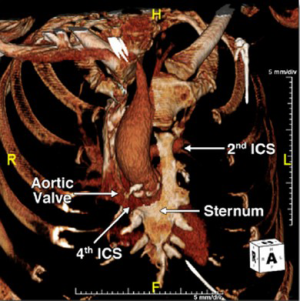
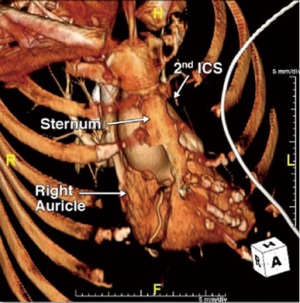
The preoperative imaging also allows the surgeon to plan their cannulation and cardioprotection strategy. Figure 1 shows that there is room for high central cannulation, which would be away from the level of the aortic valve. Induction cardioplegia is administered through an antegrade cannula in the proximal ascending aorta, either as a single dose solution or followed by intermittent maintenance doses directly into the coronary ostia depending on the surgeon’s choice of cardioplegia. Figure 3 shows that the aortic valve is positioned slightly to the right of the midline, which is ideal for a right paramedian incision. In this case, however, there was insufficient room for the ascending aortic cannula and thus we were prepared for femoral arterial and venous access.
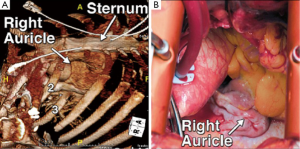
Some concerns include the cost of CT imaging, as well as the fact that surgeons are not typically reimbursed for their own imaging interpretations. CT scans are not currently required as a standard of care for patients being evaluated for aortic valve surgery. However, patients with bicuspid valve disease often get them in order to exclude associated aortopathy. Elderly patients with heavy atherosclerotic burdens may get them to confirm the safety of aortic cannulation. Other patients have a CT scan available preoperatively for a variety of reasons, including workup of lung pathologies or chest symptoms. Any of these scans, including those obtained at an outside hospital, can be reconstructed using off-line post-processing software to provide excellent detail of the aortic valve and surrounding structures.
Preoperative imaging for other intra-thoracic procedures
There is a growing appreciation for the aortopathy that may be associated with bicuspid aortic valve in up to 50% of patients. These patients are often young and free of atherosclerotic disease in their aorta. They are usually excellent candidates for combined aortic valve and ascending aortic repair. The MIAVS approach is an excellent option for these patients, including those for whom total root replacement and/or hemi-arch reconstruction with circulatory arrest is indicated. If the imaging demonstrates feasible exposure with more a more anterior origin of the branch vessels, even total arch replacement with first stage elephant trunk or frozen elephant trunk reconstruction can be performed safely through a mini exposure. For patients with a repairable insufficient bicuspid valve and root dilatation, we will recommend a valve-sparing root replacement with valve reimplantation (modified David’s procedure), and typically, this has not been performed through a mini incision.
The concept of using 3D reconstructions is not unique to minimally invasive aortic valve surgery. Multiple thoracic surgeries are facilitated by the use of preoperative 3D reconstructions. Once the surgeon becomes accustomed to this notion, he becomes much more versatile in his operative approaches. For instance, a median sternotomy or a clamshell can be used for a bilateral lung transplant. The former is much easier to heal from, but the latter provides better exposure. A preoperative reconstructed CT can help tailor the approach to the individual patient’s anatomy. Left ventricular assist device (LVAD) exchanges or explants are performed through a subcostal approach or a median sternotomy. The former is less traumatic but the latter provides better exposure. A preoperative scan can predict the best approach depending on the relation of the ventricular apex and outflow graft to the costal margin. Surgeons and cardiologists now routinely use 3D reconstructions for preoperative planning of TAVR (11). Similarly, preoperative imaging has been shown to optimize port placement and decision making in robotic heart surgery (12). A surgeon should not struggle to make the patient’s anatomy conform to their preferred operative approach; rather, the patient’s anatomy as predicted by preoperative imaging, should dictate the correct exposure.
Conclusions
Since the late 90s, minimally-invasive sternotomy has been adopted by many surgeons as the preferred approach for aortic valve and ascending aortic surgery. Yet minimally invasive incisions may limit the surgeon’s exposure, resulting in greater risk, longer operating time and a 2-4% conversion rate (3-5,13). In an analysis of 1,193 intended MIAVS at our institution, we found a 2.8% conversion rate, mostly due to the position of the heart within the chest or other unusual anatomy (13). If the valve is not positioned optimally for any particular approach, it is unwise to incur these increased risks. Unless a surgeon takes the time to understand the anatomy preoperatively, he/she may not appreciate the challenges of a particular exposure until the incision is made. With the addition of CT to the preoperative work-up, we have almost completely eliminated open conversions. In the current era of sophisticated imaging capabilities, there is no role for exploratory cardiac surgery.
The future directions report by Baumgartner et al. from the National Heart, Lung, and Blood Institute (NHLBI) stated that “in the future, computer-enhanced imaging techniques should allow surgeons to simulate surgical procedures and help assess the relative efficacy of alternative surgical approaches” (14). In order to realize these ambitions, we have to start becoming accustomed to this technology now. Just as a cardiac angiogram is ordered and reviewed by all surgeons prior to any coronary case, a review of the preoperative CT with 3D reconstructions should be done to optimize the performance of valve surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Svensson LG, D’Agostino RS. Minimal-access aortic and valvular operations, including the “J/j” incision. Ann Thorac Surg 1998;66:431-5. [PubMed]
- Svensson LG, Nadolny EM, Kimmel WA. Minimal access aortic surgery including re-operations. Eur J Cardiothorac Surg 2001;19:30-3. [PubMed]
- Malaisrie SC, Barnhart GR, Farivar RS, et al. Current era minimally invasive aortic valve replacement: techniques and practice. J Thorac Cardiovasc Surg 2014;147:6-14. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Cosgrove DM 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg 1998;65:1535-8; discussion 1538-9. [PubMed]
- Loor G, Desai MY, Roselli EE. Pre-operative 3D CT imaging for virtual planning of minimally invasive aortic valve surgery. JACC Cardiovasc Imaging 2013;6:269-71. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [PubMed]
- Ammar R, Porat E, Eisenberg DS, et al. Utility of spiral CT in minimally invasive approach for aortic valve replacement. Eur J Cardiothorac Surg 1998;14 Suppl 1:S130-3. [PubMed]
- Glauber M, Miceli A, Bevilacqua S, et al. Minimally invasive aortic valve replacement via right anterior minithoracotomy: early outcomes and midterm follow-up. J Thorac Cardiovasc Surg 2011;142:1577-9. [PubMed]
- Schoenhagen P, Tuzcu EM, Kapadia SR, et al. Three-dimensional imaging of the aortic valve and aortic root with computed tomography: new standards in an era of transcatheter valve repair/implantation. Eur Heart J 2009;30:2079-86. [PubMed]
- Bergmann P, Huber S, Segl H, et al. Cardiac MR in robotic heart surgery for preoperative identification of the target vessel and precise port placement--a theoretical model. Thorac Cardiovasc Surg 2003;51:204-10. [PubMed]
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012;144:852-858.e3.
- Baumgartner WA, Burrows S, del Nido PJ, et al. Recommendations of the National Heart, Lung, and Blood Institute Working Group on Future Direction in Cardiac Surgery. Circulation 2005;111:3007-13. [PubMed]





