Complications and conversions in minimally invasive aortic valve surgery
Introduction
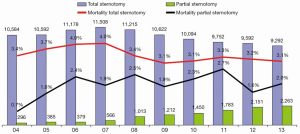
Since the emergence of limited skin incisions for aortic valve surgery in the late 1990s, several modifications have been described (1). These include upper hemisternotomy in a T- or J-shape manner, lower partial ministernotomy, right anterior or parasternal minithoracotomy, and full sternotomy with a small skin incision. However, while limited access offers advantages of minimized surgical trauma (less bone damage, pain and blood loss), the direct surgical field of view is also restricted and only permits visualization and manipulation on the cardiac situs. Consequently, the procedure requires more detailed preoperative planning and imaging, closer cooperation with anesthetists and pump technicians, good communication and teamwork with the assistant surgeon and the scrub nurses, and additional exposition tools. Furthermore, the surgeon must be well educated and experienced in managing intraoperative challenges. These circumstances have increased the complexity of the procedures, consequently limiting the uptake of minimally invasive aortic valve replacement (MIC-AVR) in most countries (Figure 1).
Here we describe key steps of the minimally invasive procedure, focusing on potential pitfalls, and recommending safeguards and solutions (see also Table 1).
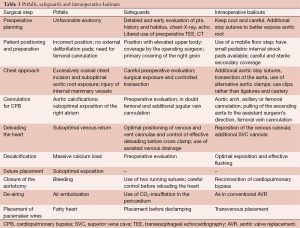
Full table
Safeguards and pitfalls
The following rules have to be kept in mind:
- Never work under pressure;
- Inform the whole team about your strategy, and be communicative;
- Never accept suboptimal surgical results as a cost of a limited approach;
- In a complex case, ask for assistance early on from an experienced surgeon;
- Use intraoperative transesophageal echocardiography (TEE) as a standard measurement.
Preoperative planning and imaging
Depending on organizational structures of the hospital, surgeons either know their patient from early on or just one day before surgery. Regardless, the patient must be seen by the operating surgeon as soon as possible, as patient characteristics play a more important role in a minimally invasive approach, compared to full sternotomy. Particular attention should be paid to body habitus (e.g., obesity, funnel chest), medical history (e.g., previous chest surgery, trauma) as well as aortic valve and root anatomy (e.g., degree of calcification, bicuspid valve, rheumatic disease, concomitant mitral calcifications). Thus, all available images should be evaluated as soon as possible. In case of doubt, a preoperative TEE and computed tomography can help to gain maximal information (1). In particular, the latter is a standard imaging modality for minimal-invasive aortic surgery in many surgical units for example, to identify the optimal intercostal space in case of a right lateral mini-thoracotomy.
In case of unforeseen and unfavorable anatomy during surgery, it is important to remain calm and ask for help from an experienced surgeon if necessary. In particular, a more anterior/posterior than lateral position of pulmonary and aortic root and caudal position of the aortic annulus, as well as fatty right ventricular outflow tract musculature are challenging to approach, but can be more easily managed using additional stay sutures.
Most minimally-invasive AVR procedures are more time-consuming than full sternotomy, prolonging the procedure by 10-30 min in experienced hands (2-5). This extension in time should be considered during patient and surgeon selection and communicated with the team members to ameliorate any time pressures.
Patient positioning and preparation
For optimal preparation and induction of anesthesia, it is very important to inform all team members about the chosen minimally-invasive approach as early as possible. Routine patient preparation should include the adherence of external defibrillation pads far away from the skin incision. In case of ineffectiveness of the external pads, a pair or sterile pediatric internal pads should be available.
The insertion of the TEE probe is strongly recommended before incision. Depending on the strategy used to drain the venous return, the groin is cleaned and covered. In most patients, it helps to angulate the operation table to better expose the groin. This is especially important for patients who do not require full sternotomy or have a large body habitus, as their upper bodies need to be elevated.
Chest approach
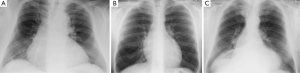
One of the most crucial steps in MIC-AVR procedures is an appropriate approach to the pericardium, which should not be too cranial or caudal (see Figure 2A-C). An overly cranial approach poses risks in thin patients (Figure 2B,C) and those with a poststenotic aortic dilatation, as most have a more caudal aortic root with projection to the inferior sternum. For these cases, an inferior sternotomy may also be considered. In contrast, in obese patients, the heart is positioned more cranially and thus exposition is easier when using an upper hemisternotomy (Figure 2A). In the case of a right minithoracotomy, the optimal intercostal space approach can be best identified by preoperative CT.
If the approach is excessively difficult, additive stay sutures at the aortic wall, a transection of the aorta and miniaturized cross clamps may help. In rare cases, minimally-invasive surgical instruments and a videoscope may facilitate the procedure and should be available.
In all chosen approaches, injury to the mammary vessel can occur. Using a right anterior thoracotomy, some surgeons primarily expose and cut these structures. In general, clipping of the vessels is safer than using ligations or cautery. The mammary vessels are at risk regardless of whether a J- or T-shape partial sternotomy, or an oscillation or jigsaw is used. Thus, careful inspection after sternotomy and before chest closure is mandatory. In some of these patients, the pleural space may be inadvertently opened. This risk should be considered, but additional drainage of the pleura space is usually not required.
Cannulation for cardiopulmonary bypass (CPB)
Calcifications of the ascending aorta may be more of a concern in MIC-AVR procedures due to restricted opportunities for palpation and limited options to cannulate the aorta. However, with transcatheter aortic valve implantations, aortic valve surgery in severely calcified ascending aorta should be avoided and excluded before surgery. In case of regional aortic wall calcifications allowing for cross clamping, cannulation of the axillary and femoral artery or the aortic arch is a favorable option. In the latter case, a Pean clamp can be used to pull the ascending aorta downwards and to easily reach the aortic arch.
In some patients with a deep seated heart and poor visualization, it is difficult to expose and cannulate the right atrial appendage. For such patients, central cannulation is facilitated by pulling the ascending aorta towards the assistant surgeon. In these challenging cases, it is helpful to provide a second suture around the cannula before removing the venous cannula to avoid bleeding. The venous cannula should be of appropriate size, but not oversized. From experience, a 28 F flexible cannula is adequate. There are different ways to divert the cannula out of the chest: (I) through an extra incision at the lower thoracic aperture (can be used for the chest drainage later on) or (II) directly at the upper or lower skin incision point. From the authors’ experience, the latter concept allows for better venous drainage, especially when the cannula is positioned right lateral to the ascending aorta.
A very suitable alternative, however, is to use a peripheral femoral vein cannula, which can even be performed percutaneously. Routinely, a 23 or 25F multistage venous cannula is used, which can be placed with the tip in the superior vena cava (SVC) under TEE control. An additional jugular vein or superior vein cannula may also be placed to optimize venous return, especially in bigger patients. When using femoral cannulation, the exposition of the aortic root is improved, but the procedure is burdened with significant extra costs and a potential risk of infection and groin complications. TEE is mandatory for a safe cannula placement.
For venting, three options are used routinely via: (I) the pulmonary artery; (II) the right upper lung vein or (III) directly through the aorta in the left ventricle.
Unloading the heart
In some patients, unloading of the heart is hindered by suboptimal venous return, as well as impaired exposition of the aortic root in a loaded right heart. Moreover, increased pulmonary venous return makes the procedure difficult or even impossible. This can be avoided by careful positioning of the venous cannula from the very beginning, before cross clamping. From the authors’ experience, vacuum-assisted venous drainage is very effective and should be routinely used. However, if deloading is ineffective, the cannula should be repositioned before continuing with the procedure. In case of increased pulmonary venous return and subsequent ineffective venting through the pulmonary trunk, the vent can be positioned directly in the left ventricle or by an extra incision through the roof of the left atrium.
Decalcification
Resection and decalcification of the stenotic aortic valve can be demanding when access is limited and root anatomy is small, as this hinders root exposure. In this situation, aortic wall or commissural stay sutures may help improve visualization of the entire annulus. Use of a headlight and an inverse Trendelenburg position of the patient are also helpful. In general, removal of very bulky calcium is the most difficult step and should be performed carefully to prevent calcium fragments from getting lost. As in a conventional full sternotomy procedure, this step should be completed by extensive saline flushing.
Valve suture placement
Placement of annular U-sutures follows the same rules as in standard procedures. Making three initial commissural sutures can further optimize exposition.
Closure of aortotomy
To avoid bleeding after declamping, it is recommended to use two continuous suture lines for aortotomy closure.
De-airing
Because of limited manipulation and de-airing of the heart, it is essential to stop the vent as early as possible to avoid coronary or brain air embolism, which can complicate the peri- and postoperative course. After closure of aortotomy and before declamping, it is important to ventilate the lungs and load the heart, and to remove the air through an aortic needle vent or similar. A very effective option, especially in minimally-invasive valve surgery, is to insufflate the operation field from the time of aortic incision onwards with carbon dioxide gas, which has higher solubility in blood. For de-airing during the reperfusion period, it is also helpful to ventilate the lungs and to let the heart eject blood, which can be verified by a discrete pulsatile arterial pressure line. TEE helps to assess the effectiveness of de-airing manoeuvers and confirm the absence of air microbubbles.
Placement of pacemaker wire
Effective placement is particularly important and depends on the pacemaker wire system used. Achieving ventricular capture can be challenging especially in right ventricles with significant adiposity. In such cases, the wire can be placed during cardiac ischemia to reach more inferior parts of the right ventricle or even the anterolateral part of the left ventricle. If needed, a transvenous pacing wire can be placed through the jugular (preferred) or femoral vein.
Conversions in MIC-AVR
Conversion of a MIC-AVR to full sternotomy is not only a defeat for the surgeon and the team, but also creates trauma to the patient. Depending on center experience, it occurs in 0.8% to 8.0% with 3-4% in almost all series (1-9). Reasons described include poor exposition; bleeding from the right ventricle, aortotomy or mammary vessels; and low cardiac output. All these complications can be minimized by a controlled and strictly protocol-driven procedure. However, in contrast to conversions of other minimally-invasive cardiac surgery procedures (e.g., minimally-invasive mitral valve surgery, OPCAB, MIDCAB or transcatheter valve implantation), conversion of a MIC-AVR is comparably easy and safe, but invariably leads to longer operation and CPB times (4). In the case of partial sternotomy, the incision can be extended to a full sternotomy. In the case of right minithoracotomy, the incision can be alternatively enlarged and a transverse sternotomy can be performed. To be able to convert at any time, the saw has to be left sterile on the table. Refixation of the transverse sternotomy can be done easily with one or two extra wires without complications in almost all patients.
Comments
As demonstrated above, MIC-AVR has its drawbacks at any time during the procedure with the potential of prolonged surgery and conversion. Thus the whole team has to be open-minded and aware of all particular pitfalls. If MIC-AVR is not the routine approach in a particular center, the minimally invasive approach should be communicated as early as possible to allow all team members to be optimally prepared. However, from our experience, it helps when MIC surgery is accepted as the standard approach in a particular center or at least for a particular surgeon. In the case of any intraoperative drawbacks, it is important that the team remains calm and solves the problem.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Plass A, Scheffel H, Alkadhi H, et al. Aortic valve replacement through a minimally invasive approach: preoperative planning, surgical technique, and outcome. Ann Thorac Surg 2009;88:1851-6. [PubMed]
- Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006;81:1599-604. [PubMed]
- Merk DR, Lehmann S, Holzhey DM, et al. Minimal invasive aortic valve replacement surgery is associated with improved survival: a propensity-matched comparison. Eur J Cardiothorac Surg 2014. [Epub ahead of print]. [PubMed]
- Tabata M, Umakanthan R, Khalpey Z, et al. Conversion to full sternotomy during minimal-access cardiac surgery: reasons and results during a 9.5-year experience. J Thorac Cardiovasc Surg 2007;134:165-9. [PubMed]
- Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013;96:837-43. [PubMed]
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [PubMed]
- Burdett CL, Lage IB, Goodwin AT, et al. Manubrium-limited sternotomy decreases blood loss after aortic valve replacement surgery. Interact Cardiovasc Thorac Surg 2014;19:605-10. [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5.
- Tabata M, Umakanthan R, Cohn LH, et al. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur J Cardiothorac Surg 2008;33:537-41. [PubMed]





