Extent II repair of thoracoabdominal aortic aneurysm secondary to chronic dissection
Introduction
Crawford extent II thoracoabdominal aortic aneurysm (TAAA) repairs generally involve replacing the full length of the thoracoabdominal aorta, from the left subclavian artery to the infrarenal abdominal aortic bifurcation, with a synthetic graft. Because of the extensive degree of aortic replacement involved, extent II repairs have been associated with the highest levels of risk for postoperative complications (1-6). To mitigate these complications, we routinely employ a multimodal approach to organ protection during these operations (7-9). To protect the spinal cord, we use mild passive hypothermia, cerebrospinal fluid drainage, left heart bypass (LHB), sequential cross-clamping, and selective reimplantation of intercostal or lumbar arteries (7,8,10,11). We intermittently deliver cold crystalloid solution to the kidneys to protect them from ischemic damage and prevent acute renal failure (12-14). We also deliver isothermic blood from the LHB circuit to the celiac axis and the superior mesenteric artery (SMA) to minimize ischemic times for the abdominal organs.
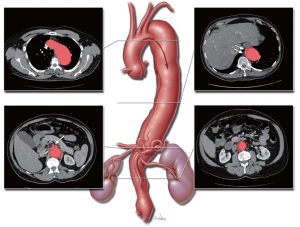
To illustrate our technique for performing extent II TAAA repair, we present a video (Video 1) of such a procedure performed in a 55-year-old man with a symptomatic TAAA associated with chronic DeBakey type III aortic dissection (Figure 1). The patient had a history of hypertension, smoking, hepatitis B and C, and hepatic cirrhosis, as well as cocaine abuse, which is a risk factor for aortic dissection (15). At the time of his referral for surgical treatment, the patient was experiencing intermittent back pain. Preoperative imaging revealed a relatively normal-sized aortic arch with a dissection membrane starting just distal to the left subclavian artery. The true lumen was narrow, and the dissection extended into the celiac axis. The aneurysm measured 6 cm in diameter, and there was a large burden of thrombus in the infrarenal region.
Operative techniques
The aneurysm was exposed through a standard thoracoabdominal inci s ion, and the ches t was entered through the 6th intercostal space. The entire thoracoabdominal aorta was exposed by performing medial visceral rotation and by circumferential division of the diaphragm. After heparin (1 mg/kg) was administered, cannulas for LHB were placed in the left inferior pulmonary vein (drainage cannula) and the distal descending thoracic aorta (inflow cannula). After LHB was initiated, the first aortic clamp was placed between the left common carotid and subclavian arteries. After a bulldog clamp was placed across the left subclavian artery, a second aortic clamp was placed across the middescending thoracic aorta, and LHB flows were increased. The isolated segment of proximal descending thoracic aorta was then opened, and the dissecting membrane was excised. All shed blood was collected via a cell-saving system and then returned to the patient through a rapid infusion system. Patent intercostal arteries were oversewn with 2-0 silk sutures.
A 24-mm Dacron graft was selected, and the proximal anastomosis was completed by using 3-0 polypropylene suture. The anastomosis was reinforced with pledgeted polypropylene mattress sutures. After the proximal anastomosis was completed, the clamp on the left subclavian artery was removed, and the aortic cross-clamp was moved down onto the graft, thereby restoring blood flow to the left subclavian artery. Left heart bypass was discontinued, the aortic cannula was removed, and the remainder of the aorta was opened down to the bifurcation. The dissecting membrane was excised to provide exposure of all intercostal, visceral, and lumbar branches.
Then, 9-Fr balloon perfusion catheters were placed in the renal arteries, the SMA, and the celiac axis. The renal arteries were infused with cold crystalloid solution, and the celiac trunk and SMA were perfused with blood from the LHB circuit (12). Suitable intercostal arteries, at the level of T10 and T11, were selected for anastomosis. An opening was created in the side of the graft, and the intercostal patch was sewn to the opening with 3-0 polypropylene suture. After this anastomosis was completed, portions of it were reinforced with pledgeted polypropylene mattress sutures. Then, another opening was made in the side of the graft adjacent to the visceral branches. In this case, all 4 vessels were incorporated into a single patch, although the left renal artery is commonly anastomosed separately. A fenestration was created in the dissecting membrane within the celiac trunk. The visceral patch was sewn to the opening with 3-0 polypropylene suture. At this point, the crossclamp was moved distally to a position below the visceral patch, thereby restoring perfusion to the intercostal and visceral vessels.
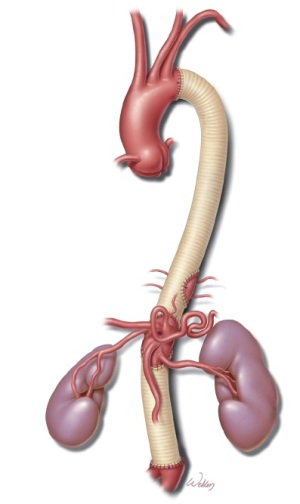
The graft was then cut to length, and the distal anastomosis was completed with 3-0 polypropylene suture. After the aortic reconstruction was completed (Figure 2), the aortic clamp was removed, restoring distal flow. Remaining intercostal and lumbar arteries were oversewn, and each anastomosis was reinforced as necessary. Protamine was administered and surgical hemostasis was achieved. After satisfactory perfusion to the liver, bowel, kidneys, and lower extremities was confirmed, the diaphragm was closed with #1 polypropylene suture. Before the wound was closed, two chest tubes were placed in the left pleural cavity, and a closed-suction drain was placed in the left retroperitoneal space.
Outcome and comments
The patient had an uneventful recovery. He was supported by a ventilator until the next morning. Cerebrospinal fluid pressure monitoring and drainage were discontinued on postoperative day 1. The patient was transferred out of the intensive care unit on postoperative day 2. His spinal cord function and renal function were normal. He was discharged home on postoperative day 7.
Although extent II TAAA repairs remain challenging procedures and are associated with substantial levels of postoperative morbidity and mortality, advances in perioperative care and surgical technique have markedly improved outcomes over the past 6 decades. As demonstrated throughout this special edition of Annals of Cardiothoracic Surgery, many different approaches to TAAA surgery have been developed in centers across the world. Although the specific strategies employed may differ, all of the approaches share the common goal of providing durable aortic repair while minimizing risks and optimizing outcomes. It is through the continued efforts of the surgical teams at these centers that further advances will be made to prevent adverse events and improve the long-term survival of patients afflicted with extensive aortic disease.
Acknowledgements
The authors thank Stephen N. Palmer, PhD, ELS, and Susan Y. Green, MPH, for editorial support, and Joseph Huh, MD, and Joseph C. Brewton for invaluable assistance with video production.
Disclosure: Dr. Coselli serves as a consultant for Vascutek Ltd., a subsidiary of Terumo Corporation.
References
- Coselli JS, LeMaire SA, Conklin LD, et al. Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2002;73:1107-15; discussion 1115-6.
- Coselli JS, LeMaire SA, Miller CC 3rd, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000;69:409-14.
- LeMaire SA, Price MD, Green SY, et al. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012; [Epub ahead of print].
- LeMaire SA, Miller CC 3rd, Conklin LD, et al. Estimating group mortality and paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2003;75:508-13.
- LeMaire SA, Miller CC 3rd, Conklin LD, et al. A new predictive model for adverse outcomes after elective thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2001;71:1233-8.
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368-70.
- Coselli JS, LeMaire SA. Tips for successful outcomes for descending thoracic and thoracoabdominal aortic aneurysm procedures. Semin Vasc Surg 2008;21:13-20.
- Vaughn SB, Lemaire SA, Collard CD. Case scenario: anesthetic considerations for thoracoabdominal aortic aneurysm repair. Anesthesiology 2011;115:1093-102.
- de la Cruz KI, LeMaire SA, Weldon SA, et al. Thoracoabdominal aortic aneurysm repair with a branched graft. Ann Cardiothorac Surg 2012;1:381-93.
- Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1999;67:1931-4; discussion 1953-8.
- Coselli JS, Lemaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
- Bhamidipati CM, Coselli JS, LeMaire SA. Perfusion techniques for renal protection during thoracoabdominal aortic surgery. J Extra Corpor Technol 2012;44:P31-7.
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8.
- Lemaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19.
- Daniel JC, Huynh TT, Zhou W, et al. Acute aortic dissection associated with use of cocaine. J Vasc Surg 2007;46:427-33.





