Think differently: trans-apical platform for TEVAR
Introduction
The endovascular treatment of thoracic aortic disease (TEVAR) is an established treatment option and indications for endoluminal therapy are rapidly expanding (1-3). Advancements in imaging provide careful pre-operative evaluation of the anatomy, making the procedural planning very accurate (4). Nevertheless, despite design improvements in endograft delivery systems, inadequate arterial access (5) and limited landing zones still represent limitations.
The apex of the left ventricle (LV) is the “front door” to the arterial vascular system and as such has gained popularity with the introduction of trans-catheter aortic valve implantation (TAVI) (6-9). In this paper we will discuss when and how trans-apical TEVAR (TaTEVAR) is a valid alternative to the conventional trans-arterial retrograde approach.
Current indications for trans-apical approach
The use of the apex of the LV as access for aortic endografting was first reported in a pig model by Grenon et al. (10) in 2009. In this paper the authors evaluated the feasibility of the antegrade trans-apical route to overcome aortoiliac tortuosity or occlusive disease. Eleven out of twelve pigs survived the experiment in a stable haemodynamic condition, despite the transient hypotension and bradycardia related to the passage of the 18-Fr sheath through the aortic valve. Most of the endografts (straight tube - Cook Medical, Inc, Bloomington, IN, USA) were deployed within 5 mm distal to the ostium of the left subclavian artery; subclavian coverage was experienced only in one case. The access at the apex was managed with a felt-pledgeted U-stitch that was passed through a Rummel tourniquet; the suture was secure in all cases. No aortic regurgitation, left ventricular dysfunction or aortic dissection was recorded. Results of this preliminary study were encouraging and they concluded that trans-apical thoracic endovascular repair might be a reasonable option in selected candidates.
A few months later, at the end of the study, the same group treated a ruptured aortic arch aneurysm endoluminally via the LV apex of a beating heart (11); the aneurysm sac was compressing the left lung. The patient was considered too high risk for open repair and had unsuitable aorto-ilio-femoral access for the standard retrograde approach; calcification of the abdominal aorta and iliac arteries prevented sewing a prosthetic conduit. Complete aortic arch debranching was performed using bilateral femoral arteries as inflow; arch branches were endo-ligated or occluded proximally to avoid type II endoleak. The apex of the LV was accessed via an anterior mini-thoracotomy at the 6th intercostal space (ICS); the aneurysm was successfully sealed, under rapid ventricular pacing (RVP), with two TAG endografts (W.L. Gore & Associates, Inc, Flagstaff, AZ, USA) deployed from the distal ascending aorta to the proximal descending via a 24-Fr sheath. Despite long operative time and considerable blood loss, the patient was stable, off inotropes and extubated on the second post-operative day; a CT scan documented a small type II endoleak, with overall good thrombosis of the aneurysm sac.
A second case was described by Szeto et al. (12); they reported on a pseudoaneurysm of the ascending aorta in a patient who had previous coronary bypass surgery. The false-aneurysm was arising from the vein graft proximal anastomosis; the vein graft was occluded so the false aneurysm could be covered with a stent-graft. The appropriate device in size was a 38 mm × 77 mm Zenith TX2 (Cook Medical, Inc), which unfortunately has a 75-cm delivery system. This device was too short to reach the sino-tubular junction when delivered in a standard retrograde fashion. A patent LIMA graft meant that subclavian artery coverage was not an option. A trans-apical approach was therefore considered to be the best option. The apex was exposed via a 5th ICS anterior thoracotomy and secured with pledgeted purse-string sutures. The endografts was successfully placed into the ascending aorta over an Amplaz Superstiff EX wire (Boston Scientific, Natick, MA, USA). A second endograft was necessary for correction of a type IA endoleak; the patient did well and the reported 6-month follow-up was satisfactory.
Uthoff et al. recently reported the successful use of the trans-apical route in another three patients with complex aortic pathologies (13). The access and management of the LV apex was conducted as described previously. Indications in this case series included: (I) repair of a type 1A endoleak of an endoluminally repaired ruptured aortic ulcer; (II) an anastomotic pseudoaneurysm of the ascending aorta following type A dissection repair; and (III) an iatrogenic type A dissection after minimally invasive mitral valve repair. All cases were successfully managed with this technique.
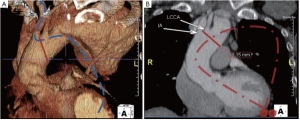
In order to overcome anatomical challenges due the geometry of the aortic arch, a different application of the trans-apical route was described by our group (14) (Video 1). After considerable TAVI experience, our group has developed a technique involving a “through-and-through” wire under constant tension going from the apex of the LV to the femoral access. This approach facilitated the precise retrograde deployment of a thoracic stent graft with a very limited proximal landing area in zone 1 (Figure 1). Although trans-brachial (15) and trans-septal (16) routes have been previously described for the “tug the wire” method, they were not suitable for our case.
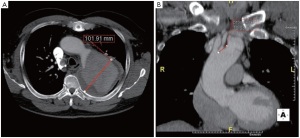
We had to deal with a Yadav type 2 aortic arch and thus a through-and-through wire from the right brachial artery would not have guaranteed the correct geometry for endograft deployment (Figure 2). The access through the apex of the LV is safe and provides a more anatomical route to the ascending aorta, avoiding the passage through the mitral valve (necessary for the trans-septal approach) with wires and catheters. Moreover, in very angulated aortic arches with a short ascending aorta, the trans-septal approach carries the risk of damaging the mitral valve and septum with the distal nose of the stent-graft delivery system. In our first case we accomplished good sealing of the aneurysm at the expense of a retrograde type A dissection that required open surgical repair. This was due to the use of an open-web configuration endograft, which we highly discourage the use of in the aortic arch. Our second and third cases (not published) were successful, confirming the safety and efficacy of the procedure.
Technical considerations
The optimal set-up for this procedure is offered by a hybrid operating suite; if not available, we found the operating theatre with a C-arm intensifier a comfortable alternative. The routine use of a trans-oesophageal echocardiogram (TOE) is mandatory; the use of ventricular pacing wires for RVP is recommended to decrease the cardiac output at the time of deployment (17). The apex of the LV can be easily identified with fluoroscopy so that the left anterior thoracotomy can be targeted to the 5th or 6th ICS. Once the pleura is opened a soft tissue retractor is introduced; we now prefer the Alexis (Applied Medical, Rancho Santa Margarita, CA, USA) over the CardioVations (Edwards Lifesciences, Irvine, CA, USA) because it provides better visibility. Once the pericardium is opened and retracted with stay sutures, we prepare the LV apex with two circular felt-pledgetted 2-0 polypropylene purse-string sutures, then secured with snuggers.
Access to the apex is gained with a 21-gauge Percutaneous Entry Thinwall Needle (Cook Medical, Inc), followed by a 10-cm 7-or 8-Fr sheath (Cordis, Johnson & Johnson, Miami, FL, USA) pushed over the wire and, through the aortic valve into the ascending aorta. At this stage, a 0.035” angled Glidewire (Terumo Medical, Somerset, NJ, USA) can be advanced through the sheath under TOE guidance into the aortic arch and the descending thoracic aorta. If antegrade deployment of the endograft is planned, the Glidewire needs to be exchanged for a stiffer wire (Amplaz Superstiff or Lunderquist, Cook Medical, Inc) using a Multipurpose 5-Fr catheter (Cook Medical, Inc). A second arterial access is needed (from a brachial or a femoral artery) for angiography. Once the appropriate endograft has been selected, the small sheath is removed and the delivery system is gently introduced via the apex, through the aortic valve and into the aorta ready for deployment. The use of RVP at the time of deployment provides controlled stability without pressure rebound. When the hardware is ready to be removed, the apex of the LV is initially controlled using RVP and then pulling on the snuggers and tying one pledgetted purse-string at a time.
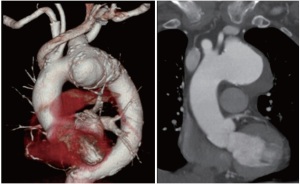
If the apex is approached for a “through-and-though” technique, a femoral access must be available; our wire of choice in these cases is the 480-cm-long, 0.035-inch MET-II guidewire (Cook Medical, Inc). In order to provide protection to the aortic arch wall, the MET-II wire can be covered with a long Flexor sheath (Cook Medical, Inc). Tension on both ends of the “through-and-though” wire associated with RVP, provides a stable platform for endograft deployment in cases with a limited landing zone and challenging anatomy (Figure 3). At the end of the procedure the chest is closed in the usual fashion over a 28-Fr chest drain.
According to the reported experience, trans-apical access does not appear to compromise the function of the aortic valve or of the LV. Crossing the aortic valve for a prolonged time with an 18-Fr sheath produces only transient hypotension, which is well-tolerated in the animal model (10) and is routinely done in TAVI cases. Despite the potential risk of neurological complications of trans-apical approach (18,19) (which remains safer then the trans-femoral approach for TAVI), no strokes have been reported so far during TaTEVAR. The idea of deflecting emboli away from the cerebral vessels has been successfully employed during TAVI (20); early results with the Embolic Deflection Device (Keystone Heart, Ltd, Herzliya, Israel) confirms a lower rate of procedure-related strokes and brain lesions on diffusion-weighted MRI. Hopefully this technology will be applicable also for TaTEVAR.
Although no access issues have been described so far during TaTEVAR, complications related to the trans-apical access site remain a concern during TAVI. Apart from bleeding from the LV apex (generally managed with extra sutures and blood transfusion and ECMO support as required), more rare complications include the formation of an LV apex pseudoaneurysm (21,22) and the presence of a late ventricular septal defect (23). Follow-up with echo is mandatory after surgical approach via the LV apex (24).
With the current TEVAR technology available, the trans-apical route limits the choice of endografts to straight tubes. In the future off-the-shelf reverse-loaded thoracic stentgrafts will be available for trans-apical delivery and this will expand the indications to include anatomy requiring tapered devices.
Conclusions
Trans-apical TEVAR is safe and feasible in complex patients with inadequate retrograde access. Furthermore, the LV apex offers a short working distance between the access site and the lesion to be treated, and in some cases a better anatomical wire configuration and tension control around the curvature of the aortic arch. Overall, trans-apical delivery combined with RVP provides a stable platform for endograft deployment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Coady MA, Ikonomidis JS, Cheung AT, et al. Surgical management of descending thoracic aortic disease: open and endovascular approaches: a scientific statement from the American Heart Association. Circulation 2010;121:2780-804.
- Gopaldas RR, Huh J, Dao TK, et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg 2010;140:1001-10.
- Desai ND, Pochettino A, Szeto WY, et al. Thoracic endovascular aortic repair: evolution of therapy, patterns of use, and results in a 10-year experience. J Thorac Cardiovasc Surg 2011;142:587-94.
- Nienaber CA, Kische S, Skriabina V, et al. Noninvasive imaging approaches to evaluate the patient with known or suspected aortic disease. Circ Cardiovasc Imaging 2009;2:499-506.
- Parmer SS, Carpenter JP. Techniques for large sheath insertion during endovascular thoracic aortic aneurysm repair. J Vasc Surg 2006;43:62A-8A.
- Rodés-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat Rev Cardiol 2011;9:15-29.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006;114:591-6.
- Ye J, Cheung A, Lichtenstein SV, et al. Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg 2006;131:1194-6.
- Blumenstein J, Van Linden A, Arsalan M, et al. Transapical access: current status and future directions. Expert Rev Med Devices 2012;9:15-22.
- Grenon SM, MacDonald S, Sidhu RS, et al. Successful ventricular transapical thoracic endovascular graft deployment in a pig model. J Vasc Surg 2008;48:1301-5.
- MacDonald S, Cheung A, Sidhu R, et al. Endovascular aortic aneurysm repair via the left ventricular apex of a beating heart. J Vasc Surg 2009;49:759-62.
- Szeto WY, Moser WG, Desai ND, et al. Transapical deployment of endovascular thoracic aortic stent graft for an ascending aortic pseudoaneurysm. Ann Thorac Surg 2010;89:616-8.
- Uthoff H, Garcia-Covarrubias L, Samuels S, et al. Transapical endovascular aortic repair to treat complex aortic pathologies. Ann Thorac Surg 2012;93:1735-7.
- Ramponi F, Vallely MP, Stephen MS, et al. Transapical wire-assisted endovascular repair of thoracic aortic dissection. J Endovasc Ther 2011;18:350-4.
- Ishimaru S, Kawaguchi S, Koizumi N, et al. Preliminary report on prediction of spinal cord ischemia in endovascular stent graft repair of thoracic aortic aneurysm by retrievable stent graft. J Thorac Cardiovasc Surg 1998;115:811-8.
- Kölbel T, Rostock T, Larena-Avellaneda A, et al. An externalized transseptal guidewire technique to facilitate guidewire stabilization and stent-graft passage in the aortic arch. J Endovasc Ther 2010;17:744-9.
- Pornratanarangsi S, Webster MW, Alison P, et al. Rapid ventricular pacing to lower blood pressure during endograft deployment in the thoracic aorta. Ann Thorac Surg 2006;81:e21-3.
- Stortecky S, Windecker S, Pilgrim T, et al. Cerebrovascular accidents complicating transcatheter aortic valve implantation: frequency, timing and impact on outcomes. EuroIntervention 2012;8:62-70.
- Eggebrecht H, Schmermund A, Voigtländer T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129-38.
- Onsea K, Agostoni P, Samim M, et al. First-in-man experience with a new embolic deflection device in transcatheter aortic valve interventions. EuroIntervention 2012;8:51-6.
- Kammler J, Steinwender C, Leisch F. False left ventricular apical aneurysm--a rare complication after transapical aortic valve replacement. J Invasive Cardiol 2011;23:534-5.
- Miranda N, Araji OA, Gutiérrez-Martín MA, et al. Spontaneous closure of pseudoaneurysm after transapical aortic valve implantation. Ann Thorac Surg 2011;92:729-31.
- Massabuau P, Dumonteil N, Berthoumieu P, et al. Left-to-right interventricular shunt as a late complication of transapical aortic valve implantation. JACC Cardiovasc Interv 2011;4:710-2.
- Nietlispach F, Eckstein F, Seeberger M, et al. Closure of apical access site after transapical, transcatheter paravalvular leak closure. Can J Cardiol 2012;28:516.