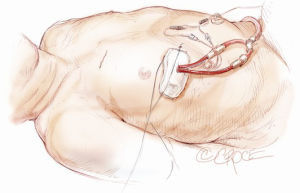
Minimally invasive aortic valve replacement: the “Miami Method”
Introduction
Aortic stenosis is the most common valvular disorder in the Western world, with a prevalence of 2% in patients over the age of 65 years to more than 4% in those greater than 85 years old (1). While disease progression is gradual, in asymptomatic patients with a peak systolic velocity ≥4 m/s, the probability of remaining free of cardiac events, including cardiac death or aortic valve surgery, is 80% at 1 year, 63% at 2 years, and 25% at 5 years (2).
Aortic valve replacement (AVR) remains the standard of care in symptomatic severe aortic stenosis and is also recommended for asymptomatic patients with left ventricular dysfunction or in those undergoing other cardiac surgery (3). However, studies have shown that up to 40% of elderly patients over the age of 70 are denied aortic valve surgery based on age and higher risk profiles (4). Advances in transcatheter aortic valve implantation have provided an alternative to inoperable high-risk aortic stenosis patients, with 1-year mortality rates reduced by at least a third compared to standard medical therapy (5-8).
First performed by Navia (9) and Cohn et al. (10), minimally invasive valve surgery has been shown to reduce morbidity (10-16) and decrease mortality in high-risk population such as the elderly and the obese, when compared with standard median sternotomy surgery (17,18). Herein, we describe our approach of minimally invasive AVR performed via a right anterior thoracotomy approach.
Operative technique
Preparation
A Swan-Ganz catheter and radial arterial line are inserted.
Exposition
Patients are placed in the supine position and undergo anesthetic induction and intubation with a single lumen endotracheal tube. A transesophageal echocardiography (TEE) probe is placed and a thorough echocardiographic evaluation is performed.
Operation
A femoral platform is the preferred method utilized to establish cardiopulmonary bypass. A 2-3 cm longitudinal incision is made above the left inguinal crease. Limited dissection and identification of the lymphatics is performed in order to decrease the risk of groin complications. A 5-0 Prolene (Ethicon, Cincinnati, OH) purse string suture is placed on the femoral artery and vein. After heparinization, a Seldinger technique is utilized to cannulate the femoral vessels. The femoral artery is cannulated with a 15-19 French arterial cannula (Biomedicus, Medtronic, Minneapolis) and the femoral vein is cannulated with a 25 French venous cannula (Biomedicus, Medtronic, Minneapolis). With the aid of TEE guidance, the venous cannula is placed in the superior vena cava. This is essential to assure adequate drainage (Figure 1). Routine pre-operative computed tomography (CT) screening for aortic pathology or vascular disease is not performed. If peripheral vascular disease is suspected at the time of femoral cannulation or grade 4 or 5 atherosclerotic disease is evident by TEE, axillary artery cannulation or central aortic cannulation is performed.
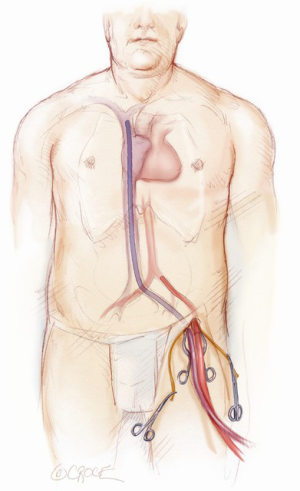
Cardiopulmonary bypass is initiated using a closed membrane oxygenator and a roller pump. The patient’s temperature is allowed to drift. Venous drainage is augmented with vacuum assistance applying negative pressures of 30-70 mmHg as needed to decompress the right heart.
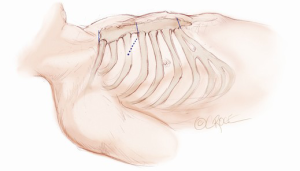
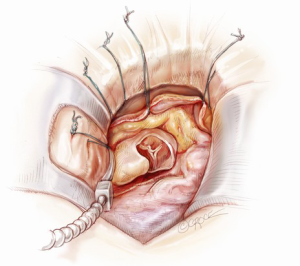
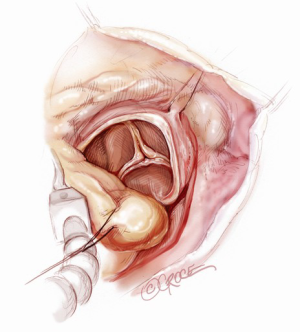
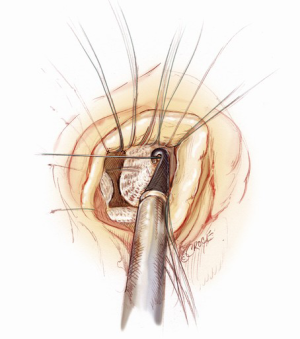
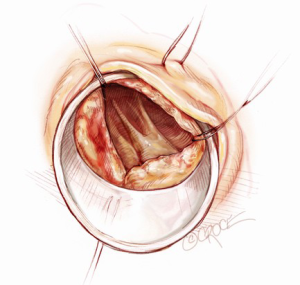
A 5-6 cm transverse incision is then made over the right 2nd intercostal space. A basic external landmark is to locate the midway point of an imaginary line draw from the suprasternal notch to the lowest portion of the body of the sternum. The xiphoid is not included. The incision should be made from this point laterally (Figure 2). The right internal mammary and vein are ligated and transected. The cartilage of the inferior rib is always transected and later reattached. Avoiding transection and stretching the interspace will create a large chest wall defect and potentially paradoxical chest wall motion (Figure 3). A soft tissue retractor (Alexis Wound Retractor, Applied Medical, Rancho Santa Margarita, CA, USA) and an intercostal rib spreader (Intercostal Rib Spreader, Miami Instruments, Miami, FL, USA) provide additional exposure. The pericardium is opened over the aorta and extended down towards the inferior vena cava (IVC). The pericardium is opened superiorly following the greater curvature of the aorta. The pericardium is not opened up to its attachment to the aorta as this will limit the ability to place stay sutures. Carefully planned placement of pericardial stay sutures is the key to obtaining adequate exposure of the aorta and valve. The sutures are placed on the pericardium over the pulmonary artery, aortopulmonary window and above the aorta. These sutures are crucial to provide the necessary exposure. Sutures are continually placed to further pull the aorta into the operative field. A CT scan of the chest to determine whether the aorta lies to the left of the midline is not performed nor necessary. This finding is not a contraindication to perform this procedure. A retrograde cardioplegia cannula (Edwards Lifesciences, Minneapolis) is inserted directly through the right atrial appendage. This will facilitate exposure of the aorta when the appendage is retracted. If a retrograde cardioplegia cannula is not utilized, a snare is created with a number 2 silk and a red rubber catheter. This is passed through the chest tube incision and the appendage snared and pulled downward. A left ventricular vent (Ventricular Vent, Medtronic, Minneapolis, MN, USA) is then placed via the right superior pulmonary vein (Figure 4). Both are passed through the chest tube incision. This allows for a smaller incision to be performed and also decreases clutter in the operative field (Figure 5). Trans-incisional direct aortic cross clamping is then performed utilizing a flexible and retractable shaft cross clamp (Cygnet, Vitalitec, Plymouth, MA). Dissection between the aorta and pulmonary artery is not necessary and is a potential source of bleeding. One dose of antegrade modified Del Nido cardioplegia solution is given to establish electromechanical arrest of the heart (Figure 6). This 4:1 blood to modified Del Nido cardioplegia has allowed at least 90 minutes of myocardial protection before any additional doses are required. If retrograde cardioplegia cannulation is not possible or is not considered necessary and additional doses of cardioplegia are needed, this is delivered directly into the coronary ostia. Carbon dioxide is infused into the operative field at 2 L/min (Figure 7). A transverse aortotomy is made at the level of the fat pad on the aorta. It is important to make the aortotomy at least 2 cm from the cross clamp in order to facilitate closure. A silk suture is placed on the superior aspect of the aorta to provide retraction and exposure of the aortic valve (Figure 8). Thereafter, sutures are placed on the commissures to provide additional retraction and exposure of the aortic valve. Resection of the valve leaflets and debridement of the annulus are carried out under direct vision utilizing standard techniques. All procedures are performed with specially designed long shafted minimally invasive instruments (Vitalitec, Plymouth, MA, USA). A specially designed aortic wall retractor (Aortic Cuff, Miami Instruments, Miami, FL, USA) is inserted and fixed in place with the suture supporting the commissure between the left and non-coronary cusp. This facilitates visualization of the annulus and suture placement (Figure 9). The aortic wall retractor is removed after the valve sutures are all placed. The valve is sized after suture placement. After the valve is delivered onto the annulus, the three commissural valve sutures are initially tied. A knot setter (Knot Setter, Miami Instruments, Miami, FL, USA) is utilized for tying the valve sutures. Coordination and practice with an assistant will allow tying five knots with each suture in approximately 8 seconds (Figure 10). After closure of the aortotomy, before removing the cross clamp, a ventricular pacing wire was placed on the inferior wall of the right ventricle. This cannot be performed once the clamp is released.

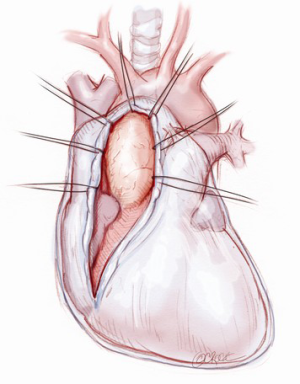
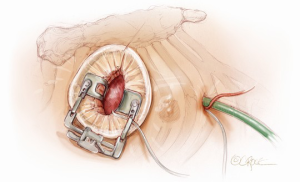
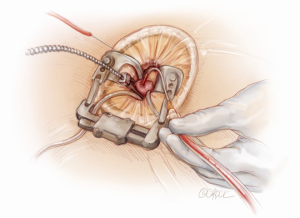
In patients undergoing AVR, with previous coronary artery bypass surgery and a patent left internal mammary graft, we use moderate hypothermia (28 °C) with one induction dose of antegrade modified Del Nido cardioplegia. Thereafter, retrograde cardioplegia is delivered in a continuous fashion throughout the procedure. We do not identify nor dissect the left internal mammary artery (LIMA) pedicle. In the setting of a patent LIMA with a partially patent native vessel, a constant stream of blood return from the left main will obscure the operative field. In these cases we place a #10 French red rubber catheter directly into the left main connected to a pump suction to aspirate the blood.
Completion
TEE is utilized to assess the post-operative results as well as removal of air from the heart. This is performed with a needle placed in the root of the aorta (Figure 11). The heart is not directly manipulated during air removal maneuvers. If needed, external compression of the chest wall is performed to agitate the air bubbles. After discontinuing cardiopulmonary bypass and administering one half of the protamine, the femoral venous cannula is removed. When the entire dose of protamine is given and the patient is hemodynamically stable, the arterial cannula is removed and the purse string suture tied. A Blake chest tube (Ethicon, Cinncinati, OH, USA) is positioned in the posterior pleural space and another in the pericardial space. For pain relief, an On-Q pain system (I-Flow, Kimberly Clark Healthcare Company, Lake Forest, CA, USA) with two catheters is placed freely into the pleural space and 0.25% bupivicaine is administered for 72 hours. The chest tubes and catheters along with the pacing wire are passed through the chest tube incision (Figure 12). The pericardium is usually not closed, although if possible, it should be considered. Closing the pericardium will limit adhesions of the lung to the heart for future interventions. Thereafter, a large pericostal suture is placed in a figure of eight fashion to approximate the ribs. The transected rib is re-attached to the sternum with a number 2-0 Vicryl suture placed in a figure of eight fashion as well. The thoracotomy incision is closed in the routine fashion.
Comments
Clinical results
At our institution from November 2008 to April 2014, we have performed 3,738 cardiac surgeries, of which 2,344 were performed by a single surgeon utilizing a minimally invasive approach. During this time, 857 isolated primary AVRs were performed. This did not include minimally invasive double valve procedures nor concomitant aortic valve and ascending aortic/hemi arch surgeries. The mean age of these patients was 72±12 years, and there were 509 (59.4%) males and 348 (40.6%) females.
The median aortic cross clamp and cardiopulmonary bypass times were 83 minutes [interquartile range (IQR), 71-100] and 110 minutes (IQR, 95-132), respectively. Cerebrovascular accidents were noted in 8 (0.93%) patients. The median hospital length of stay was 6 days (IQR, 4-8), and the 30-day mortality was 14 (1.63%).
Advantages
Cardiac surgeons should pursue and perform mini thoracotomy, minimally invasive aortic valve surgery in order to offer a “true” minimally invasive platform. Avoiding a sternal splitting procedure decreases the potential of bleeding as well as the need for transfusions with its associated complications. Other advantages include improved chest wall stability which allows increased mobility as well as an accelerated recovery. This particular procedure and method is very competitive to transcatheter procedures in high risk, elderly, frail and obese patients. To my knowledge, to date, there are no robust studies comparing this particular approach to transcatheter AVR’s, whether it be randomized or case-matched. Furthermore, expanded applications of this minimally invasive method addressing the ascending aorta as well as multivalve pathology will allow an extensive subset of patients to be treated. We will be approaching the panacea of minimally invasive aortic valve surgery once technical expertise and proficiency is further advanced with sutureless or rapid deployment valves in combination with a mini thoracotomy approach.
Caveats
This method has definitive advantages, although there is a learning curve. There is a learning curve associated with any cardiac surgical procedure, despite what experienced surgeons now consider routine and simple. In order to overcome the conceptual “learning curve”, surgeons need to consider this the standard of care in isolated AVR surgery and make it reality. This will provide a clinical benefit to our patients, as well as advance our specialty. Adoption rates are low due to complacency with conventional sternotomy techniques and the rapidly changing health care environment. This should not deter cardiac surgeons from providing advanced minimally invasive techniques to our patients. Whether an AVR is performed via a sternotomy or a minithoracotomy, the size of the aortic annulus does not change. One needs to become comfortable working in a smaller space and become proficient with the use of long shafted instruments. Developing additional techniques and maneuvers within ones comfort zone will provide the necessary exposure. The devoted surgeon interested in developing a minimally invasive program needs to experience live case demonstrations, review videos of the procedure, read technical manuscripts, consider being proctored and finally begin the journey!
Acknowledgements
Dr. Chris Mihos and Dr. Andres Pineda for their contribution to this publication.
Disclosure: Dr. Joseph Lamelas recieves honoraria for lectures and courses with Medtronic, St. Jude and On-Q and is a partner in Miami Instruments. All surgeries were performed by a single surgeon, Dr. Joseph Lamelas.
References
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. [PubMed]
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290-5. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [PubMed]
- Bouma BJ, van den Brink RB, Zwinderman K, et al. Which elderly patients with severe aortic stenosis benefit from surgical treatment? An aid to clinical decision making. J Heart Valve Dis 2004;13:374-81. [PubMed]
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90. [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [PubMed]
- Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg 1996;62:1542-4. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Mihos CG, Santana O, Lamas GA, et al. Incidence of postoperative atrial fibrillation in patients undergoing minimally invasive versus median sternotomy valve surgery. J Thorac Cardiovasc Surg 2013;146:1436-41. [PubMed]
- Valdez GD, Mihos CG, Santana O, et al. Incidence of postoperative acute kidney injury in patients with chronic kidney disease undergoing minimally invasive valve surgery. J Thorac Cardiovasc Surg 2013;146:1488-93. [PubMed]
- Mihos CG, Santana O, Lamas GA, et al. Outcomes of right minithoracotomy mitral valve surgery in patients with previous sternotomy. Ann Thorac Surg 2011;91:1824-7. [PubMed]
- Pineda AM, Santana O, Lamas GA, et al. Is a minimally invasive approach for re-operative aortic valve replacement superior to standard full resternotomy? Interact Cardiovasc Thorac Surg 2012;15:248-52. [PubMed]
- Santana O, Reyna J, Benjo AM, et al. Outcomes of minimally invasive valve surgery in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2012;42:648-52. [PubMed]
- Pineda AM, Santana O, Reyna J, et al. Outcomes of reoperative aortic valve replacement via right mini-thoracotomy versus median sternotomy. J Heart Valve Dis 2013;22:50-5. [PubMed]
- Lamelas J, Sarria A, Santana O, et al. Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann Thorac Surg 2011;91:79-84. [PubMed]
- Santana O, Reyna J, Grana R, et al. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg 2011;91:406-10. [PubMed]