Minimally invasive aortic valve surgery: state of the art and future directions
Introduction
Aortic valve disease is the most common valvular heart disease in developed countries and its incidence is likely to increase with age (1). Aortic valve replacement (AVR) through a full sternotomy (FS) is the conventional approach for the treatment of aortic valve disease and data reported from the Society of Thoracic Surgeon (STS) database have shown a dramatically in-hospital mortality reduction from 3.4% in 1997 to 2.6% in 2006 for isolated AVR (2). Despite these excellent results, there has been an increasing number of cases performed via minimally invasive aortic valve replacement (MIAVR). This approach has now become an established alternative to FS in order to reduce the “invasiveness” of the surgical procedure, while maintaining the same efficacy, quality and safety of a conventional approach.
Definition, surgical approaches and rational for MIAVR
The STS database defines minimally invasive cardiac surgery as “any procedure not performed with a FS and cardiopulmonary bypass (CPB) support” (3,4). The only procedure precisely represented by this definition is transcatheter aortic valve implantation (TAVI). Nevertheless, in 2008, a scientific statement from the American Heart Association defines minimally invasive cardiac surgery “a small chest wall incision that does not include the conventional FS” (5). We will use the latter definition, since the term “minimally invasive cardiac surgery” should not be related to a specific procedure, but rather a “concept” or a “philosophy” that requires an operation-specific strategy aiming to reduce the degree of surgical invasiveness (3).
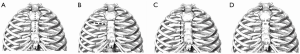
The most common minimally invasive approach is the partial upper ministernotomy (MS), followed by the right anterior minithoracotomy (RT). Other less-invasive techniques include a right parasternal approach from the second to the fourth costal cartilages and transverse sternotomy (Figure 1).
MIAVR was first performed through a 10 cm right parasternal approach in 1996 at the Cleveland Clinic Foundation by Cosgrove and Sabik (6). This technique involved removal of the second, third and fourth costal cartilages, sacrificing the right mammary artery. The major limitation of this technique was a high incidence of lung herniation, which may be physiologically disturbing and cosmetically disfiguring, often requiring a second operation for repair. This approach was soon abandoned in favour of the MS approach (7).
Compared with conventional surgery, MIAVR has been shown to provide faster recovery, shorter hospital stay, improved cosmesis and less wound infection. In addition, MIAVR has shown to improve postoperative respiratory function due to the preservation of sternum, and reduction of postoperative pain, blood loss and blood transfusions related to the reduction of surgical dissection, as well as facilitating reoperation at a later date, as part of pericardium remains closed. Finally, MIAVR requires fewer rehabilitation resources and consequently associated costs are reduced (7-11).
Ministernotomy (MS) and minithoracotomy (RT)
The MS approach represents the most common technique used for MIAVR. The MS sternotomy approach is achieved through 6 to 10 cm midline vertical skin incision, performing a partial J sternotomy at the third to fifth intercostal space or a V-shaped MS at the level of the second or third intercostal space (3,12). A number of retrospective studies and several reviews have shown that MS can be performed safely without the risk of death or other major complications (8-11). In a meta-analysis of 4,586 patients, Brown et al. demonstrated that MIAVR by the way of MS was associated with shorter ventilation time, intensive care unit stay and hospital stay as well as less blood loss within 24 hours compared with conventional surgery, although patients undergoing MS had longer cross-clamp and CPB time (8). No difference was found in terms of postoperative atrial fibrillation, stroke and sternal complications (8). Similar results were reached by others (9-11). A meta-analysis of randomized trials showed that MS significantly reduces the length of stay in the cardiac ICU (11). Other short term benefits were deduction in blood loss and length of hospital stay (11). Finally, in a meta-analysis of 4,667 patients undergoing any MIAVR approach Murtuza et al. reported benefits in perioperative mortality, intensive care unit stay, total hospital stay and ventilation time in the MIAVR group compared to conventional surgery, although, once again, operative times were longer (9).
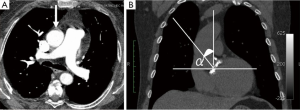
Data reported from these studies have focused mainly on upper MS; few studies have described the potential advantages of MIAVR using a RT approach (9,13-15). In 2011, we reported our first experience with MIAVR using the RT approach and showed excellent surgical results in terms of mortality, morbidities, and patient satisfaction (13). In contrast to MS, all patients scheduled for RT should undergo computed tomography scan without contrast enhancement to evaluate the anatomic relationship among the intercostal spaces, ascending aorta, and aortic valve. Patients are suitable for RT only if the following criteria are met: (I) at the level of main pulmonary artery, the ascending aorta is rightward (more than one half located on the right in respect to the right sternal border); (II) the distance from the ascending aorta to the sternum do not exceed 10 cm; (III) the α angle (angle between the angle midline and the inclination of ascending aorta) should be >45°. Exclusion criteria for the RT approach are prior cardiac surgery, history of right-sided pleuritis, and aortic root dilatation (Figure 2) (13). The surgical technique has been described elsewhere (13). Briefly, MIAVR by way of RT is performed through a 5 to 7 cm skin incision placed at the level of the second intercostal space without rib resection. After sacrificing the right internal thoracic artery, a soft tissue retractor is inserted into the thoracotomy and direct aortic cannulation is performed using flexible cannulas. Afterward, a percutaneous cannula is inserted through the femoral vein into the right atrium to achieve the venous drainage under transesophageal echocardiographic guidance and Seldinger’s technique. After establishing vacuum-assisted CPB, a left ventricular vent is placed through the right superior pulmonary vein. Then, the ascending aorta is then clamped and antegrade cardioplegic solution is given into the aortic root or selectively into the coronary ostia.
From January 2005 and June 2010, 192 consecutive patients underwent isolated MIAVR through the RT approach (13). Overall mortality was 1.6% and the rate of intraoperative conversion was 1.6%. Interestingly, although the cross-clamp and CPB time were longer than that of the standard approach, the incidence of postoperative atrial fibrillation and blood transfusion were 18% and 16%, respectively. The median of the length of stay was five days and discharge home was 90%. Finally, 96% of patients believed had an aesthetically pleasing scar and 95% were back to their normal activities within four weeks. The potential advantages of RT approach were demonstrated after comparing patients undergoing conventional surgery (16). Specifically, 138 patients undergoing RT were matched to a FS group using propensity score analysis. The overall in-hospital mortality was 0.7%, with no difference between the two groups. MIAVR via RT was associated with a lower incidence of postoperative atrial fibrillation (18.1% vs. 29.7%) and blood transfusions (18.8% vs. 34.1%) compared to FS. In addition, patients in the RT group had a shorter mechanical ventilation time (6 vs. 8 hours) and postoperative length of stay (5 vs. 6 days). No difference was found in terms of late mortality at median follow-up of 30 months (range, 17-54 months). Finally, among patients undergoing MIAVR, we found that patients receiving a RT approach had better outcomes than those receiving MS in terms of lower postoperative atrial fibrillation (19.5% vs. 34.2%), shorter ventilation time (median 7 vs. 8 hours) and hospital stay (median 5 vs. 6 days) (17). Interestingly in all our studies, a low rate of atrial fibrillation was found to be associated with the RT approach. This might be related to the preservation and reduced trauma of the sternum, which translates into reduced postoperative pain and improved respiratory function. The smaller pericardial incision and the absence of manipulation of the right atrium for the venous drainage may also be responsible for reducing the inflammatory response, thus triggering less atrial fibrillation. Therefore, shorter ventilation time, reduced blood loss, as well as the low rate of atrial fibrillation and blood transfusions, translate in a shorter hospital stay, faster recovery and fewer rehabilitation resources (17).
Criticisms
Although MIAVR has showed excellent results, there are still criticisms regarding this approach. First, the advantages of the MIAVR appears to be more related to improved cosmetic results rather than better clinical outcomes, as the majority of published studied are retrospective and randomized trials have not showed any potential advantages (8-11). However, these randomized trials are statistically underpowered and not based on contemporary patient cohorts. Future prospective randomized studies are very difficult to design and conduct, given that MIAVR has been shown to have equivalent results to the standard FS approach, but with the reduced surgical invasiveness which patients prefer. The second criticism relates to the morbidity associated with peripheral cannulation, which may cause wound infection, pseudoaneurysms and neurological events. In order to avoid these complications, our preference is to cannulate the ascending aorta, which allows a more direct and physiological flow. We have recently demonstrated that the retrograde perfusion is an independent risk factor for neurological complications such as stroke and postoperative delirium (18). Third criticism regards the costs related to the minimally invasive surgical instrumentations. Although all the instruments and devices used for MIAVR are more expensive, we strongly believe the less rate of postoperative complications, the shorter hospital stay and the faster recovery translate in less resources in the healthy system and therefore lower costs. However, this hypothesis should be evaluated with a well-designed study. Fourth, minimally invasive surgery is not “surgeon friendly’ as it is more complex and technically challenging. It entails a distinct learning curve because of the deeper operative field, limited working space for the exposure and implantation of the prosthetic valve, as well as the use of new equipment and methods (19). With regards to this problem, we have evaluated a single surgeon’s learning curve (M.G.) with RT using the cumulative sum (CUSUM) analysis (20). Surgical failure was defined as the occurrence of one or more of the following events: perioperative death, intraoperative conversion to FS, perioperative myocardial infarction, arrhythmias, postoperative complete atrioventricular block, acute renal failure, neurological events, reoperation for bleeding, prolonged ventilation time and surgical wound infection. From the first 100 patients undergoing RT, we found a low risk of cumulative failures in absence of learning curve effect. Therefore, patients undergoing MIAVR through RT are not exposed to an increased operative risk during the surgeon’s initial experience. However, we strictly recommend following our CT scan rules and beginning with select low risk patients. Once the surgeon feels confident with this new technique, he should consider sicker patients who will have the greater benefits. Finally, MIAVR is associated with longer CPB and cross-clamp time, and this has raised some concerns regarding its safety in elderly and high risk patients, because they are well known risk factors for adverse outcomes after cardiac surgery (21,22). Despite these excellent results, we confirm that patients undergoing MIAVR via RT had longer operative times than those who received a FS (13,16,17). This is a limitation of our approach, suggesting that exposure and implantation of the prosthetic valves are more challenging than the conventional approach. However, the use of the sutureless devices has reduced the operative times, further facilitating and standardizing the MIAVR approach.
Sutureless MIAVR
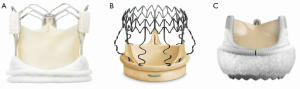
In the recent years, three different sutureless or rapid deployment aortic valves have been introduced in Europe for use in both conventional AVR and MIAVR operations—the Enable™ Valve System (Medtronic, Minneapolis, MN, USA), the Perceval S™ Valve System (Sorin Biomedica Cardio Srl, Sallugia, Italy), and the Edwards Intuity™ Valve System (Edwards Lifesciences, Irvine, CA, USA, Figure 3). Favorable clinical outcomes with the use of these valves have been reported in several studies (23-25). However, few studies report clinical outcome of patients who underwent MIAVR approach using sutureless valves. Up to date, we described the largest case series of patients who underwent MIAVR through RT and MS, reporting excellent hemodynamic results, postoperative outcomes, and short postoperative operative times (26). Specifically, our operative mortality was 0.7% and compared with our previous studies of MIAVR with stented valves, we found a 38% and 40% reduction in the cross-clamp and CPB time in the RT group and 43% and 35% in the MS group, suggesting that sutureless valves can facilitate and standardize the surgical procedure. In addition, we found a very low incidence of paravalvular leakage (1.8%), a frequent complication of TAVI procedure. It has been shown that significant paravalvular leakage is associated with higher risk of late mortality (27,28). To avoid paravalvular leakage using sutureless valves, we strongly recommend removing all the eccentric calcifications and creating a complete decalcification of the aortic annulus. In our experience this has reduced dramatically the number of paravalvular leakage in our first series (26). In a recent study of patients undergoing MS approach and sutureless devices, Santarpino et al. showed better outcomes in the sutureless group, suggesting that the combination of a MIAVR associated with a sutureless valve may be the first-line treatment for high risk patients considered in the gray zone between TAVI and conventional surgery (29,30). TAVI has shown excellent results when compared to standard therapy (31). However, controversies exist when compared to a surgical population (27,32,33). Several studies concluded that TAVI is likely ineffective in reducing early and midterm all-cause mortality versus surgical AVR (16,32). In addition, transcatheter procedures are associated with greater incidence of neurological events and paravalvular leakages, which are well known risk factors for worse survival (33). Furthermore, cost-effectiveness analysis studies of TAVI conclude that it is inappropriate to consider reimbursement of TAVI for high-risk operable patients due to the similar mortality rate at one year and the higher proportion of postoperative complications (34,35). However, these studies focused on a group of patients undergoing conventional surgery with stented-sutured valves. Therefore, in the light of the good hemodynamic performances of sutureless, the low rate of postoperative complications and paravalvular leakages, we believe that the association of MIAVR and sutureless valves may be a valid alternative to the new growing TAVI technology in high-risk “operable patients”. These data require further confirmation by well-designed prospective randomized trials.
Conclusions
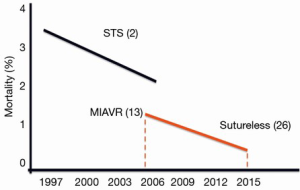
MIAVR performed through RT or MS is a safe procedure associated with excellent postoperative outcomes, in terms of mortality, morbidity, shorter hospital stay and faster recovery. Although, cross-clamp time and CPB times are longer than conventional surgery, the use of sutureless or fast deployment valves may increase the usage of MIAVR approach among surgeons, as they make these accesses technically easier and more reproducible. This combination may further improve postoperative outcomes (Figure 4). With the introduction of sutureless valves, the operative mortality of MIAVR patients has decreased from 1.6% to 0.7% in a seven year period (13,26). In the light of these results, we believe that MIAVR with a sutureless prosthesis might be considered an “alternative” to TAVI procedure for high-risk “operable patients”. However, a randomized trial is required to confirm our hypothesis.
Acknowledgements
Disclosure: Dr. Glauber M and Ferrarini M have disclosure with Sorin.
References
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66. [PubMed]
- Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [PubMed]
- STS National Database Spring 2003, Executive Summary. Duke Clinical Research Institute, Durham, NC (2003).
- Rosengart TK, Feldman T, Borger MA, et al. Percutaneous and minimally invasive valve procedures: a scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2008;117:1750-67. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Shekar PS, Cohn LH. Minimally invasive aortic valve surgery. In: Cohn LH. eds. Cardiac surgery in the Adult, 3rd ed. New York: McGraw-Hill Professional;2007:957-62.
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-9.e5.
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [PubMed]
- Khoshbin E, Prayaga S, Kinsella J, et al. Mini-sternotomy for aortic valve replacement reduces the length of stay in the cardiac intensive care unit: meta-analysis of randomised controlled trials. BMJ Open 2011;1:e000266. [PubMed]
- Karimov JH, Santarelli F, Murzi M, et al. A technique of an upper V-type ministernotomy in the second intercostal space. Interact Cardiovasc Thorac Surg 2009;9:1021-2. [PubMed]
- Glauber M, Miceli A, Bevilacqua S, et al. Minimally invasive aortic valve replacement via right anterior minithoracotomy: early outcomes and midterm follow-up. J Thorac Cardiovasc Surg 2011;142:1577-9. [PubMed]
- Plass A, Scheffel H, Alkadhi H, et al. Aortic valve replacement through a minimally invasive approach: preoperative planning, surgical technique, and outcome. Ann Thorac Surg 2009;88:1851-6. [PubMed]
- Ruttmann E, Gilhofer TS, Ulmer H, et al. Propensity score-matched analysis of aortic valve replacement by mini-thoracotomy. J Heart Valve Dis 2010;19:606-14. [PubMed]
- Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg 2013;145:1222-6. [PubMed]
- Miceli A, Murzi M, Gilmanov D, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg 2014;148:133-7. [PubMed]
- Murzi M, Cerillo AG, Miceli A, et al. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: a propensity score analysis on 1280 patients. Eur J Cardiothorac Surg 2013;43:e167-72. [PubMed]
- Cooley DA. Minimally invasive valve surgery versus the conventional approach. Ann Thorac Surg 1998;66:1101-5. [PubMed]
- Murzi M, Cerillo AG, Bevilacqua S, et al. Traversing the learning curve in minimally invasive heart valve surgery: a cumulative analysis of an individual surgeon's experience with a right minithoracotomy approach for aortic valve replacement. Eur J Cardiothorac Surg 2012;41:1242-6. [PubMed]
- Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104-9. [PubMed]
- Ranucci M, Frigiola A, Menicanti L, et al. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis 2012;21:732-9. [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [PubMed]
- Martens S, Sadowski J, Eckstein FS, et al. Clinical experience with the ATS 3f Enable® Sutureless Bioprosthesis. Eur J Cardiothorac Surg 2011;40:749-55. [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: Early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [PubMed]
- Miller DC, Blackstone EH, Mack MJ, et al. Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg 2012;143:832-843.e13.
- Santarpino G, Pfeiffer S, Jessl J, et al. Sutureless replacement versus transcatheter valve implantation in aortic valve stenosis: a propensity-matched analysis of 2 strategies in high-risk patients. J Thorac Cardiovasc Surg 2014;147:561-7. [PubMed]
- D'Onofrio A, Messina A, Lorusso R, et al. Sutureless aortic valve replacement as an alternative treatment for patients belonging to the "gray zone" between transcatheter aortic valve implantation and conventional surgery: a propensity-matched, multicenter analysis. J Thorac Cardiovasc Surg 2012;144:1010-6. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013;2:10-23. [PubMed]
- Takagi H, Niwa M, Mizuno Y, et al. A meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg 2013;96:513-9. [PubMed]
- Neyt M, Van Brabandt H, Devriese S, et al. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open 2012;2. [PubMed]
- Indraratna P, Ang SC, Gada H, et al. Systematic review of the cost-effectiveness of transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2014;148:509-14. [PubMed]