Best surgical option for thoracoabdominal aneurysm repair - the endovascular approach
Management of thoracoabdominal aortic aneurysms (TAAA) remains a great challenge for cardiovascular surgeons. Astonishingly, since the series reported by DeBakey and colleagues in 1956 (1), concerns have remained the same: visceral ischemia, limb ischemia, spinal ischemia and respiratory insufficiency.
By definition, the thoracic endovascular aortic repair (TEVAR) option should, in theory, eliminate both visceral ischemia and respiratory insufficiency, as visceral ischemia time is minimised and there is no opening of the diaphragm and chest, which are the main reasons for prolonged intubation and respiratory insufficiency in the open repair setting.
The applicability and anatomic limitations of these devices and techniques are always a concern. In our experience, cases considered inappropriate for endovascular repair are highly unusual and rarely found; in fact, there is enormous anatomical consistency in visceral vessel anatomy, as we mentioned in our first publication on this subject (2).
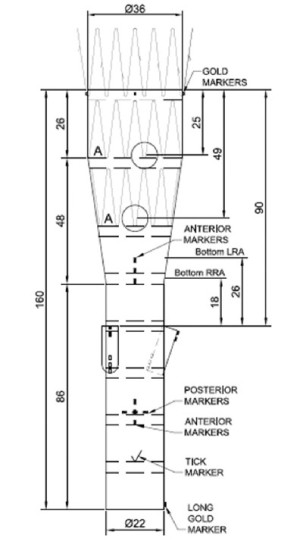
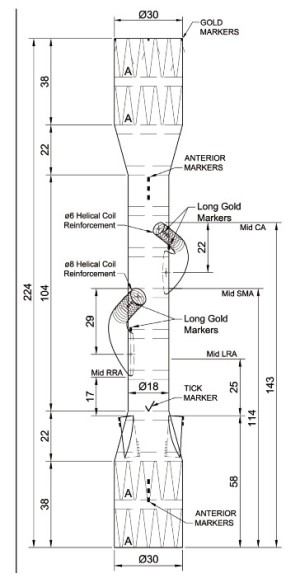
An off-the-shelf device for TAAA repair is now a reality, as has been reported recently by Chuter (3) (Figure 1), although we believe his results are more optimistic than those we would expect in a real-world scenario. Thus far, we have only worked with custom-made devices, and based on our series, we foresee that on average, a third of the patients still require a custom-made device.
Figures 2-10 provide examples of a number of custom designs we have used in cases with challenging anatomy in the last 5 years, to illustrate the variety of real-world anatomy.




In this issue of the Annals of Cardiothoracic Surgery, we review our experience with the endovascular repair of TAAAs (Table 1).
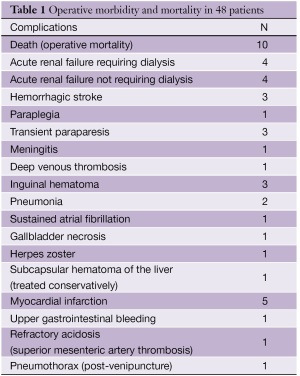
Full table
Interestingly, although the aforementioned premises are partly true (especially concerning visceral ischemia and respiratory insufficiency), the mortality rates reported herein are unfortunately much higher than the best rates reported by centers of excellence dedicated to this challenging subject or even from other centers dedicated to TEVAR (4-6). We analyzed our data extensively in an attempt to understand these outcomes and were able to detect many potential factors that can explain them, at least in part.
First, ours was a consecutive series of patients. All patients, regardless of clinical risk, were offered this option, many times as a compassionate measure.
Second, we have to operate in different hospitals due to insurance company requirements. This exposes patients to very different realities, e.g., many intensive care teams see such cases only once a month or even as rarely as once a year. We find this scheme—which is, of course, related to country-specific health policies—particularly suboptimal, as the learning curve should include not only surgeons but also the nursing staff and intensive care team. Ideally, these cases should be concentrated in selected centers dedicated to aortic surgery.
An interesting finding is that almost 50% of deaths in the series were due to myocardial infarction.
Another very interesting point observed in this series was the fact that events resulting in death occurred after patients had already recovered from anesthesia, and no major spinal cord deficits were observed, which is very much a point in favor of TEVAR.
In our experience, paraplegia rates have been relatively low (2%) which is in keeping with most current publications. Our strategy for prevention of paraplegia is essentially to keep systolic blood pressure above 120 mmHg and spinal fluid pressure below 12 cmH2O. Although we have not used the “perfusion sac branch” to prevent paraplegia as proposed by Krassi in previous reports, we think it can be a good adjunct for this purpose (7,8).
Overall, we believe that a point has been reached where comparison between TEVAR and open procedures is no longer the most important aspect regarding TAAA therapy. Our major target now is to improve the outcomes of TEVAR and disseminate its practice, as it is clear that both patients and surgeons would not prefer an overtly morbid procedure that involves opening the chest, abdomen, and diaphragm, which may have devastating impact on the patient's quality of life. Whereas endovascular treatment provides outcomes that are at least similar to those of open techniques and has broad technical potential given the rapid pace of material development.
Acknowledgements
Disclosure: Marcelo Ferreira is a proctor for Cook Medical in Latin America.The other authors declare no conflict of interest.
References
- Debakey ME, Creech O Jr, Morris GC Jr. Aneurysm of thoracoabdominal aorta involving the celiac, superior mesenteric, and renal arteries; report of four cases treated by resection and homograft replacement. Ann Surg 1956;144:549-73.
- Ferreira M, Lanziotti L, Monteiro M. Branched devices for thoracoabdominal aneurysm repair: early experience. J Vasc Surg 2008;48:30S-36S; discussion 36S.
- Chuter T, Greenberg RK. Standardized off-the-shelf components for multibranched endovascular repair of thoracoabdominal aortic aneurysms. Perspect Vasc Surg Endovasc Ther 2011;23:195-201.
- Verhoeven E, Tielliu IF, Zeebregts CJ, et al. [Results of endovascular repair of TAAA in the first 50 patients]. Zentralbl Chir 2011;136:451-7.
- O’Brien N, Sobocinski J, d’Elia P, et al. Fenestrated endovascular repair of type IV thoracoabdominal aneurysms: device design and implantation technique. Perspect Vasc Surg Endovasc Ther 2011;23:173-7.
- Mastracci TM, Eagleton MJ. Endovascular repair of type II and type III thoracoabdominal aneurysms. Perspect Vasc Surg Endovasc Ther 2011;23:178-85.
- Harrison SC, Agu O, Harris PL, et al. Elective sac perfusion to reduce the risk of neurologic events following endovascular repair of thoracoabdominal aneurysms. J Vasc Surg 2012;55:1202-5.
- Lioupis C, Corriveau MM, Mackenzie KS, et al. Paraplegia prevention branches: a new adjunct for preventing or treating spinal cord injury after endovascular repair of thoracoabdominal aneurysms. J Vasc Surg 2011;54:252-7.