The choice of mitral annuloplastic ring—beyond “surgeon’s preference”
Introduction
Annuloplasty is a fundamental component in mitral valve repair. According to Carpentier’s original concepts, an annuloplastic ring is essential for the following three reasons: (I) to restore the size and shape of the native annulus; (II) to prevent future annular dilatation; and (III) to provide functional annular support. Logically, Carpentier designed his annuloplastic ring to remodel the annulus into the shape it takes in the systolic phase (1). Because the entire mitral valvular structure experiences the highest stress during this phase, this particular configuration may restore maximal leaflet coaptation (1). Over the past two decades, although the choice of annuloplastic ring has been the focus of extensive investigation and debate, to date it still largely remains a matter of “surgeon’s preference” rather than an evidence-based selection. In fact, only a handful of prospective randomized clinical trials had ever been conducted and existing knowledge on this particular subject is far from conclusive (2).
The impact of an annuloplastic ring
To properly address the issue of ring choice, in-depth understanding of the related pathophysiology is crucial. Miller’s group at Stanford University (3-6) and Gorman’s group at University of Pennsylvania (7-9) have made substantial contributions to understanding of the mechanisms involved in mitral annuloplasty. These researchers’ observations of the 3-dimensional (3D) dynamics of the mitral valve showed that without ring implantation, the mitral annular area varied throughout the cardiac cycle. Regardless of the type of ring implanted, the mitral annular area and shape were almost fixed after ring annuloplasty (3,4). In particular, ring annuloplasty markedly reduced the mobility of the central posterior leaflet, so that valve closure was largely a single anterior leaflet process with a frozen posterior buttress (4,5). Moreover, the minimum mitral area observed during the cardiac cycle without a ring was nearly 50% larger than the “fixed” annular area with a semi-rigid or flexible ring (4,5). Hence, regardless of ring choice, mitral annuloplasty may unavoidably reduce the mitral annular area and prohibit the motion of the posterior leaflet. Based on such considerations, some surgeons elected to avoid implanting a ring whenever possible (10-14).
Few randomized studies so far have provided convincing long-term data comparing the durability of annuloplasty with and without prosthetic rings. However, several large series from centers of excellence have demonstrated the efficacy of the ring in preventing the late recurrence of mitral regurgitation. DiBardino et al. (15) and Gillinov et al. (16,17) confirmed a significant advantage of ring annuloplasty over no-ring repair in terms of the durability of reconstruction. In a recent Cleveland Clinic report involving 3,074 patients with isolated posterior leaflet prolapse, the lack of a prosthetic ring annuloplasty was an independent risk factor for the postoperative return of mitral regurgitation (18). On a separate note, these observations indeed underline the potential disadvantage in some recently developed percutaneous mitral valve “repair” techniques where the intervention is focused only on the leaflets.
The Cleveland Clinic series (18) predominantly used the Cosgrove flexible band, which was primarily designed for preserving mitral annular dynamics (19). It has been suggested that the saddle shape and the sphincter mechanism of the mitral valve can be preserved for up to five years after implantation of this band (20). The Mayo Clinic group also reported good mid-term outcomes for the routine application of a flexible but “standard-sized” (unmeasured) posterior annuloplasty band (21). However, there is still a lack of real long-term data on the durability of the flexible band.
The routine use of a flexible partial band may carry some potential drawbacks. It appears logical to suspect that due to pannus formation with subsequent fibrotic changes and calcifications (22), the band may become more rigid over time. Thus, it remains doubtful as to whether the annular saddle shape and sphincter mechanism can be maintained beyond the mid-term. Additionally, few would argue that the flexible band is unlikely to retain a saddle shape if the native annulus was flattened in the first place. Most importantly, the C-shaped flexible band is inherently unable to restore the 3:4 (vertical:transverse) annular physiological relationship originally proposed by Carpentier et al. (1).
The importance of saddle-shaped annuloplasty
Although the mitral annular saddle shape (Figure 1) has long been recognized and carefully studied, its clinical relevance was not fully appreciated until recently. Using real-time 3D transesophageal echocardiography (TEE), we (23) observed that the annular saddle shape was closely related to the scale of leaflet prolapse, the extent of chordal elongation, and the frequency of chordal rupture, and consequentially that it determined the evolution of mitral regurgitation in patients with mitral valve prolapse. This in vivo human evidence highlighted the significance of the annular saddle shape in disease progression. Recently, we (24) also found that obvious mitral annular disjunction (defined as a separation between the atrial-mitral valve junction and ventricular attachment) was evident in 20 out of 96 (21%) patients with myxomatous mitral valve prolapse. Compared with the remaining 76 patients, the patients with mitral annular disjunction had significantly greater commissural width, shorter annular height, but similar annular anteroposterior diameter, area and circumference, resulting in a flatter and more elliptical annular shape. Annular unsaddling, defined as annular height to commissural width ratio (AHCWR) less than 15%, was significantly more prevalent in the group with mitral annular disjunction than without (90% vs. 46%, χ2=9.674, P=0.002). Mitral annular disjunction was also associated with significantly greater leaflet billowing volume and longer chordal lengths. Our findings suggested that mitral annular disjunction may contribute to the change of mitral valvular 3D geometry, with reduced annular height and loss of the saddle shape.
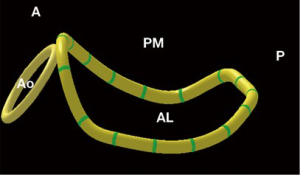
Importantly, serial investigations by the Gorman group have discovered that the saddle-shaped annulus reduces strain on the mitral leaflet, particularly at the P2 site (25-27). On the one hand, accumulating clinical evidence indicates that such a saddle shape is distorted in patients with myxomatous or ischemic mitral regurgitation (9,23,24). On the other hand, Jensen and colleagues (28-30) showed that a saddle-shaped annuloplasty ring can provide a more favorable and uniform annular force distribution when compared to the flat ring. The saddle ring can maintain the medial alignment of the papillary muscles to facilitate proper leaflet coaptation (29,30). Therefore, by diminishing mitral leaflet strain and improving leaflet coaptation geometry, saddle-shaped annuloplasty may enhance repair durability. Again, to maintain the annular saddle shape, it is sensible to choose a complete annuloplastic ring rather than a flexible C-shaped band.
“Saddle ring for all patients” versus a tailored selection
We, like many other groups, had adopted different annuloplasty strategies over the past decades, ranging from “no ring whenever possible” at the early phase of our valve repair program, to the current practice of using a complete saddle-shaped ring almost exclusively. In the past ten months, however, we have observed that the native mitral annular saddle shape could often be well preserved in patients suffering from a relatively short duration of mitral regurgitation. Such a phenomenon is particularly true in those young patients with acute infective endocarditis. Based on our own experience, a semi-rigid ring rather than a saddle-shaped ring can provide more reliable repair results in this particular patient subgroup. Indeed, Carpentier’s statement remains inspiring even 30 years later: “one may define the aim of a valve reconstruction as restoring normal valve function rather than normal valve anatomy” (31). For a durable mitral valve repair, it remains controversial whether a saddle-shaped annuloplasty should be applied to every patient. In fact, it was proposed that mitral annular dynamic changes (i.e., “flexibility”) may largely be limited following a saddle-shaped annuloplasty using either a Physio-II ring or a Rigid Saddle ring (32).
For the purpose of tailored selection of an annuloplastic ring, we (33) prospectively performed 3D-TEE in 31 normal subjects (the control group) and in 88 patients with degenerative mitral regurgitation prior to surgical mitral valve repair. Zoomed real-time 3D-TEE images of the entire mitral complex, including annulus, leaflets, papillary muscles and the aortic valve, were acquired. Quantitative morphological analysis of the mitral valve was performed with custom software (QLAB MVQ, Philips Healthcare, Andover, MA, USA). The reconstructed valve was displayed as a color-coded 3D-rendered surface representing a topographical map. Measurements of the key 3D geometric parameters of the mitral annulus were automatically generated. AHCWR was calculated as a surrogate of the annular saddle-shape. Group comparisons used one-way ANOVA or the χ2 test as appropriate. Post hoc comparisons used LSD tests.
Main echocardiography findings
We observed that AHCWR ranged from 15-33% (mean ± SD =24%±5%) in the normal control group. Annular flattening, defined as AHCWR <15%, was evident in 71% of patients with anterior leaflet or bi-leaflet prolapse and in 53% of those with isolated posterior leaflet prolapse. Moreover, height and AHCWR were both significantly lower in the anterior- or bi-leaflet prolapse group than in the isolated posterior leaflet prolapse group, despite the annular area, circumference, commissural width and anteroposterior diameters all being similar between the two groups. Thus, we confirmed that annular flattening is common in patients with mitral valve prolapse, but the degree of such flattening appears to be more marked in those with anterior leaflet or bi-leaflet prolapse. In these patients, a saddle-shaped annuloplasty may be more important in restoring annular geometry and function.
Clinical results
Over the same four-year study period, 198 consecutive patients underwent mitral valve repair at our institution. Among them, 189 received saddle-shaped annuloplasty (including nine emergency cases with preoperative IABP and/or mechanical ventilatory support). Concomitant aortic valve replacement (n=17), tricuspid repair (n=91) or coronary artery bypass grafting (n=42) were carried out. The overall 30-day surgical mortality was 2.5% (n=5).
Ring selection (AHCWR <15%)
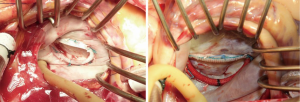
We selected the Physio-II ring (Edwards Lifesciences, Irvine, CA, USA) for patients with degenerative mitral regurgitation when true-size annuloplasty is indicated (n=131). Meanwhile, we chose the Rigid Saddle ring (St. Jude Medical, St. Paul, MN, USA) for patients with functional or ischemic mitral regurgitation (n=58) that warrants a down-sizing annuloplasty (Figure 2). Over a mean follow-up of 2.8 years, the majority of patients (97%) had less than mild to moderate (2+) mitral regurgitation. The recurrence of moderate to severe mitral regurgitation was found in only two patients in the Physio-II group and three patients in the Rigid Saddle ring group.
Ring selection (AHCWR ≥15%)
We found that a semi-rigid ring rather than a saddle-shaped ring may provide more reliable repair results in patients whose AHCWR remained within the normal range. We have chosen the Memo 3D ring (Sorin SpA, Milan, Italy) under such circumstances with satisfactory early results (n=9). Postoperative 3D-TEE confirmed that mitral annular saddle shape is well preserved in these patients (AHCWR ranged from 15-18%).
Conclusions
Taken together, the use of a saddle-shaped ring rather than a flat ring may enhance the mechanical benefits conferred by mitral annuloplasty. Aiming to preserving native mitral annular saddle shape or performing a saddle-shaped annuloplasty, we routinely use 3D TEE to measure the AHCWR prior to every mitral valve repair operation for an individualized selection of the appropriate annuloplastic ring. Indeed, the benefit of such a clinical implication is yet to be evaluated prospectively in a large patient population with adequate long-term follow-up.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Carpentier AF, Lessana A, Relland JY, et al. The "physio-ring": an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 1995;60:1177-85; discussion 1185-6. [PubMed]
- Chang BC, Youn YN, Ha JW, et al. Long-term clinical results of mitral valvuloplasty using flexible and rigid rings: a prospective and randomized study. J Thorac Cardiovasc Surg 2007;133:995-1003. [PubMed]
- Glasson JR, Komeda MK, Daughters GT, et al. Three-dimensional regional dynamics of the normal mitral anulus during left ventricular ejection. J Thorac Cardiovasc Surg 1996;111:574-85. [PubMed]
- Glasson JR, Green GR, Nistal JF, et al. Mitral annular size and shape in sheep with annuloplasty rings. J Thorac Cardiovasc Surg 1999;117:302-9. [PubMed]
- Green GR, Dagum P, Glasson JR, et al. Restricted posterior leaflet motion after mitral ring annuloplasty. Ann Thorac Surg 1999;68:2100-6. [PubMed]
- Dagum P, Timek T, Green GR, et al. Three-dimensional geometric comparison of partial and complete flexible mitral annuloplasty rings. J Thorac Cardiovasc Surg 2001;122:665-73. [PubMed]
- Parish LM, Jackson BM, Enomoto Y, et al. The dynamic anterior mitral annulus. Ann Thorac Surg 2004;78:1248-55. [PubMed]
- Levack MM, Jassar AS, Shang EK, et al. Three-dimensional echocardiographic analysis of mitral annular dynamics: implication for annuloplasty selection. Circulation 2012;126:S183-8. [PubMed]
- Jassar AS, Vergnat M, Jackson BM, et al. Regional annular geometry in patients with mitral regurgitation: implications for annuloplasty ring selection. Ann Thorac Surg 2014;97:64-70. [PubMed]
- Eisenmann B, Charpentier A, Popescu S, et al. Is a prosthetic ring required for mitral repair of mitral insufficiency due to posterior leaflet prolapse? Long-term results in 96 patients submitted to repair with no ring. Eur J Cardiothorac Surg 1998;14:584-9. [PubMed]
- Maisano F, Caldarola A, Blasio A, et al. Midterm results of edge-to-edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surg 2003;126:1987-97. [PubMed]
- Aybek T, Risteski P, Miskovic A, et al. Seven years' experience with suture annuloplasty for mitral valve repair. J Thorac Cardiovasc Surg 2006;131:99-106. [PubMed]
- Soon JL, Du X, Shine B, et al. Local suture annuloplasty for posterior mitral valve repair: 18-year experience. Asian Cardiovasc Thorac Ann 2011;19:20-6. [PubMed]
- Fundarò P, Tartara PM, Villa E, et al. Mitral valve repair: is there still a place for suture annuloplasty? Asian Cardiovasc Thorac Ann 2007;15:351-8. [PubMed]
- DiBardino DJ, ElBardissi AW, McClure RS, et al. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010;139:76-83; discussion 83-4. [PubMed]
- Gillinov AM, Cosgrove DM, Blackstone EH, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 1998;116:734-43. [PubMed]
- Gillinov AM, Tantiwongkosri K, Blackstone EH, et al. Is prosthetic anuloplasty necessary for durable mitral valve repair? Ann Thorac Surg 2009;88:76-82. [PubMed]
- Johnston DR, Gillinov AM, Blackstone EH, et al. Surgical repair of posterior mitral valve prolapse: implications for guidelines and percutaneous repair. Ann Thorac Surg 2010;89:1385-94. [PubMed]
- Cosgrove DM 3rd, Arcidi JM, Rodriguez L, et al. Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg 1995;60:499-503; discussion 503-4. [PubMed]
- Gillinov AM, Cosgrove DM 3rd, Shiota T, et al. Cosgrove-Edwards Annuloplasty System: midterm results. Ann Thorac Surg 2000;69:717-21. [PubMed]
- Brown ML, Schaff HV, Li Z, et al. Results of mitral valve annuloplasty with a standard-sized posterior band: is measuring important? J Thorac Cardiovasc Surg 2009;138:886-91. [PubMed]
- Luk A, Jegatheeswaran A, David TE, et al. Redo mitral valve surgery: morphological features. Cardiovasc Pathol 2008;17:309-17. [PubMed]
- Lee AP, Hsiung MC, Salgo IS, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation 2013;127:832-41. [PubMed]
- Jin CN, Fang F, Meng FX, et al. Mitral annular disjunction and unsaddling in myxomatous mitral valve prolapse: a 3-dimensional transesophageal echocardiographic study. Eur Heart J Cardiovasc Imaging 2014;15:ii25-51.
- Jimenez JH, Liou SW, Padala M, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J Thorac Cardiovasc Surg 2007;134:1562-8. [PubMed]
- Padala M, Hutchison RA, Croft LR, et al. Saddle shape of the mitral annulus reduces systolic strains on the P2 segment of the posterior mitral leaflet. Ann Thorac Surg 2009;88:1499-504. [PubMed]
- Vergnat M, Jackson BM, Cheung AT, et al. Saddle-shape annuloplasty increases mitral leaflet coaptation after repair for flail posterior leaflet. Ann Thorac Surg 2011;92:797-803. [PubMed]
- Jensen MO, Jensen H, Smerup M, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation 2008;118:S250-5. [PubMed]
- Jensen MO, Jensen H, Levine RA, et al. Saddle-shaped mitral valve annuloplasty rings improve leaflet coaptation geometry. J Thorac Cardiovasc Surg 2011;142:697-703. [PubMed]
- Jensen MO, Hagège AA, Otsuji Y, et al. The unsaddled annulus: biomechanical culprit in mitral valve prolapse? Circulation 2013;127:766-8. [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Ryomoto M, Mitsuno M, Yamamura M, et al. Is physiologic annular dynamics preserved after mitral valve repair with rigid or semirigid ring? Ann Thorac Surg 2014;97:492-7. [PubMed]
- Jin CN, Wan S, Wong RH, et al. Flattening of annulus saddle-shape in patients with mitral valve prolapse: comparison between posterior and anterior leaflet prolapse. Eur Heart J 2014;35 Suppl 1:746. [PubMed]





