Training in video-assisted thoracoscopic lobectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) is a minimally invasive alternative to open thoracotomy for major pulmonary resection and has been shown to have a number of benefits. VATS lobectomy is a safe and cost-effective treatment option (1,2). Compared to open thoracotomy, VATS lobectomy is associated with a reduction in post-operative pain, lower incidence of pneumonia and a shorter hospital stay, whilst maintaining equivalent oncological outcomes and long-term survival (3-9).
However, despite the perceived benefits of VATS lobectomy, the majority of lobectomies are still performed as open procedures with relatively few centres offering VATS. This may be because the VATS approach is considered to be technically demanding with the potential for catastrophic haemorrhage in the hands of an inexperienced surgeon. Furthermore, the difficulty in arranging training in VATS lobectomy has meant that the technique has been disseminated slowly.
We believe that senior trainees and consultants with ample experience of open lobectomy can be trained to perform VATS lobectomy by an experienced VATS surgeon without affecting patient outcomes. We here report the experience of a senior cardiothoracic surgical trainee undertaking a one-year fellowship with intensive exposure to VATS lobectomy under the supervision of a highly experienced consultant in a high-volume thoracic surgical unit.
Methods
A prospectively collected database of all patients undergoing VATS lobectomy in a single institution during a one-year period was reviewed. The database holds the demographic and baseline clinical characteristics of patients operated upon as well as intra-operative details and information regarding post-operative recovery and clinical outcome.
The supervising consultant has 20 years of experience of VATS lobectomy and has performed in excess of 800 major VATS resections. At the start of the fellowship the trainee had undertaken 55 open lobectomies and 75 minor VATS procedures (including lung biopsy and wedge resection) during six years of cardiothoracic surgical training, but had no previous experience of VATS lobectomy. The patients were listed for surgery according to clinical priority using our standard processes with no modification of case selection for the trainee.
Surgical technique
Our VATS lobectomy technique has been described in detail and aims to replicate the dissection used for open lobectomy whilst taking advantage of the benefits of smaller incisions. An entirely thoracoscopic approach was used for all patients. In brief, with the patient in the lateral decubitus position and the lung to be operated upon deflated, three port sites were created: a 5 cm utility incision anteriorly in the sixth or seventh intercostal space, a further 2 cm instrument port level with this in the mid-axillary line and a 2 cm camera port in the auscultatory triangle. A fissure-based posterior approach was used to access the hilum and dissect out the lobar pulmonary artery branches, pulmonary vein tributaries and bronchus, each of which were divided using an endoscopic linear stapling device. A comprehensive lymphadenectomy was performed complementing pre-operative mediastinoscopy. An intercostal drain was placed and typically removed after 24 hours if the chest radiograph and aerostasis were satisfactory. A local anaesthetic paravertebral catheter was used alongside a patient controlled morphine pump for analgesia, and patients benefited from daily physiotherapy.
Data analysis
Only the 59 procedures performed by the trainee were analysed. For the purpose of analysis, the series was divided into two groups where group 1 included the first 30 cases and group 2 consisted of the last 29 cases performed by the trainee. These two groups were compared to see whether there were any differences in operative and clinical outcomes.
Unpaired two-tailed Student t-test and Fischer’s exact (GraphPad Prism, GraphPad Software, USA) were used where appropriate. Two-sided P<0.05 was regarded as statistically significant.
Results
The trainee performed 59 of the 69 (86%) VATS lobectomy procedures undertaken during the study period. There were no differences in the patient characteristics between the two groups (Table 1). A similar range of operations was performed in both time periods (Table 2). There was no difference between the two groups in terms of the mortality rate or hospital stay. There was no significant difference in the rate of conversion to open thoracotomy or airleak persisting for more than 7 days. No patient required re-operation.
| Table 1 Patient characteristics | |||
| Group 1 | Group 2 | Total | |
| Age (mean, range, years) | 67 (52-87) | 69 (47-86) | 68 (47-87) |
| Female (%) | 19 (63%) | 15 (52%) | 34 (58%) |
| Benign disease (%) | 1 (3%) | 1 (3%) | 2 (3%) |
| There were no differences in patient characteristics between the two groups (P=NS) | |||
| Table 2 Clinical and operative outcomes | |||
| Group 1 (n=30) | Group 2 (n=29) | Total (n=59) | |
| Primary procedure | |||
| - Right upper | 8 (27%) | 11 (38%) | 19 (32%) |
| - Right middle | 3 (10%) | 4 (14%) | 7 (12%) |
| - Right lower | 2 (7%) | 3 (10%) | 5 (8%) |
| - Left upper | 8 (27%) | 3 (10%) | 11 (19%) |
| - Left lower | 5 (17%) | 1 (3%) | 6 (10%) |
| - Segmentectomy | 4 (13%) | 7 (24%) | 11 (19%) |
| Additional wedge resections | 1 (3%) | 2 (7%) | 3 (5%) |
| Conversion to open thoracotomy | 1 (3%) | 1 (3%) | 32 (3%) |
| Operation time (mean±SEM, min) | 190±6 | 186±7 | 188±5 |
| Blood loss (mean±SEM, mL) | 142±27 | 87±9 | 115±15 |
| Hospital stay (median, range, days) | 6 (3-40) | 4 (3-26) | 5.5 (3-40) |
| 30 day mortality | 1 (3%) | 1 (3%) | 2 (3%) |
| Air leak >7 days | 5 (17%) | 2 (7%) | 7 (12%) |
| There were no differences in operative or clinical outcomes between the two groups (P=NS) | |||
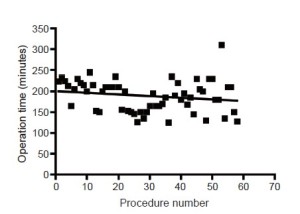
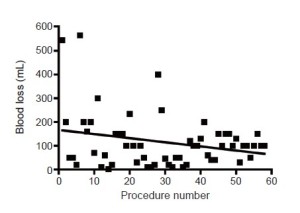
There was a gradual reduction in operating time with increasing experience (Figure 1) but this did not reach statistical significance. There was also a small reduction in operative blood loss (Figure 2) which was neither clinically nor statistically significant.
Discussion
Video-assisted thoracoscopic lobectomy has a number of potential benefits when compared to open thoracotomy but is only practiced in a few thoracic surgical centres (1-7). VATS lobectomy is regarded as a technically complex procedure. Difficulty in obtaining training in such techniques may be part of the reason why VATS lobectomy is not employed more widely. We have previously shown that the performance of a consultant who was self taught in VATS lobectomy improved rapidly over the first 46 cases he performed, and continued to improve more slowly over the remaining 184 cases in the series (10). In this paper we report on the learning curve of a senior cardiothoracic surgical trainee learning VATS lobectomy under the supervision of the same, now highly experienced, consultant. Before undertaking this fellowship the trainee had previously performed 55 open but no VATS lobectomies. Interestingly, when supervised by the experienced consultant, the learning curve was almost eliminated when measured in terms of operating time and blood loss.
The operation time reported here for the trainee is similar to that reported in contemporary series for surgeons learning VATS lobectomy, and is approximately 20-30 min longer than for experienced VATS lobectomy surgeons (10,11). It is worth noting that direct comparison of operation times with those reported in historical series is confounded by the current practice of comprehensive lymphadenectomy which was not always undertaken for cases performed during earlier time periods and which adds 30-45 minutes onto the overall operating time. However, it would seem wise when scheduling cases to anticipate a slightly longer operation time if a trainee is performing the procedure. The operative blood loss for the trainee in this series also compares favourably to that reported in other series (10,11).
Importantly, clinical outcomes including persistent airleak, hospital stay and mortality were similar to those reported in other series and did not exhibit a learning curve effect. This supports the findings of two previous papers showing that VATS lobectomy can be taught safely to both trainees and consultants (11,12).
It would seem appropriate for VATS training opportunities to be reserved for those who are likely to benefit most from them such as recently appointed consultants and senior trainees with sufficient experience of open major pulmonary resection. In other specialties, laparoscopic simulators have been shown to attenuate the learning curve for a number of procedures as well as for basic laparoscopic skills such as dissection and suturing (13-16).
Development of generic laparoscopic skills using simulators prior to starting a VATS fellowship may be advantageous, allowing trainees to capitalise on the learning opportunities presented to them. Indeed a placement in another speciality, such as general surgery in which laparoscopic procedures such as cholecystectomy and appendicectomy are better established and more widely practiced, may also be beneficial in terms of building initial laparoscopic experience prior to a VATS fellowship.
In order for more patients to benefit from the VATS approach, the issues surrounding training in VATS lobectomy require to be addressed. We believe that intensive exposure to VATS lobectomy in a high-volume centre is advantageous to training. It may, therefore, be appropriate for national training programmes to be established with fellowships in designated high-volume centres. When aiming to disseminate VATS lobectomy to other centres it is important to remember that it is not just the surgeon that requires training. Opportunities should be created for theatre nursing staff and procurement teams to visit established VATS centres to observe the theatre arrangements and receive advice regarding the recommended equipment.
In conclusion, the data presented here demonstrate that a senior cardiothoracic surgical trainee can be trained in VATS lobectomy without impacting adversely on clinical outcomes. If VATS lobectomy is to become more widely practiced, consideration should be given to the provision of VATS fellowships and the availability of adjuncts to training.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Walker WS, Pugh GC, Craig SR, et al. Continued experience with thoracoscopic major pulmonary resection. Int Surg 1996;81:255-8.
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32.
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70.
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5.
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13.
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7.
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11.
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402.
- Jiang G, Yang F, Li X, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non-small cell lung cancer. World J Surg Oncol 2011;9:170.
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9.
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20.
- Wan IY, Thung KH, Hsin MK, et al. Video-assisted thoracic surgery major lung resection can be safely taught to trainees. Ann Thorac Surg 2008;85:416-9.
- Aggarwal R, Ward J, Balasundaram I, et al. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. Ann Surg 2007;246:771-9.
- Aggarwal R, Grantcharov TP, Eriksen JR, et al. An evidence-based virtual reality training program for novice laparoscopic surgeons. Ann Surg 2006;244:310-4.
- Stefanidis D, Hope WW, Korndorffer JR Jr, et al. Initial laparoscopic basic skills training shortens the learning curve of laparoscopic suturing and is cost-effective. J Am Coll Surg 2010;210:436-40.
- Larsen CR, Soerensen JL, Grantcharov TP, et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ 2009;338:b1802.