Sutureless Aortic Valve Replacement International Registry (SU-AVR-IR): design and rationale from the International Valvular Surgery Study Group (IVSSG)
Introduction and rationale
Aortic stenosis is the most frequent valvular cardiac disease in the developed world, accounting for a pooled prevalence of 12.4% in the elderly population (1). The prognosis for symptomatic patients with severe aortic stenosis is dismal, with a one-year mortality of 30-50% (2,3). Aortic valve replacement (AVR) via median sternotomy approach, using a biological or mechanical prosthesis, has been largely shown to be safe and long-term efficacious, and thus currently represents the “gold-standard” approach for aortic stenosis treatment (4).
Despite excellent outcomes of conventional AVR over the past two decades, this surgical approach has evolved to become less invasive and to expand the boundaries of operability towards elderly patients presenting with multiple comorbidities and higher surgical risk. Firstly, minimally invasive aortic valve replacement (MI-AVR) has been introduced (5) and has slowly gained acceptance as a less traumatic alternative compared to median sternotomy (6-9). However, due to the technical challenges involved and the lack of data showing a substantial survival benefit and a reduced occurrence of major post-operative complications from MI-AVR over conventional management, this approach has not been universally adopted.
The observation that 30% of patients with severe aortic stenosis were not referred because they were deemed inoperable (10), has recently triggered the development of newer approaches and technologies. Compared to standard medical therapy, transcatheter aortic valve implantation (TAVI) has shown to provide a 26.8% absolute reduction in mortality at 3-year follow-up in inoperable patients (3,11), and has demonstrated great potential for high-risk surgical candidates (12). While the uptake and growth for TAVI has been enthusiastic and widespread in Europe and North America, concerns exist surrounding paravalvular leakage, vascular complications, stroke, post-operative Pacemaker implantation due to complete AV block, optimal access sites, long-term valve durability, and economic sustainability meaning that the optimal treatment of high-risk operable patients remains controversial and requires further long-term follow-up and critical assessment (12-15).
The rapid technological progress of innovative surgical approaches has also resulted in the natural evolution of sutureless aortic valves from conventional sutured valves. SU-AVR, by avoiding placement and tying of sutures after annular decalcification, has shown to minimize cross-clamp and cardiopulmonary bypass durations (16,17). Shortened operational durations of SU-AVR may help reduce post-operative mortality and morbidity and improve cost-effectiveness, particularly in high risk patients as well as in those undergoing complex or concomitant procedures (18).
There is a paucity of robust, evidence-based data on the role and performance of SU-AVR in minimally invasive and conventional aortic valve surgery. It is unclear how the long term outcomes of SU-AVR will compare with existing and well-accepted procedures for patients with aortic stenosis in different risk settings. A coherent and unified international collaborative effort will be necessary to provide statistically-powered multi-institutional evidence to evaluate this innovative technique and direct future avenues of research.
Sutureless aortic valves
There are three main types of sutureless aortic prostheses which are currently available on the market, including the 3F Enable (Medtronic, Minneapolis, USA), Perceval S (Sorin, Saluggia, Italy) and Intuity (Edwards Lifesciences, Irvine, USA) sutureless valves (Figure 1).

The 3F Enable Sutureless aortic valve, CE mark approved in 2009, consists of a 3F stentless aortic bioprosthesis, designed to closely mimic the function of the native aortic valve. The 3F Enable is assembled from three equal sections of equine pericardial material, fixed with glutaraldehyde and mounted on a self-expanding Nitinol frame, which fixes the device in the native annulus by virtue of outward radial force. This allows for the use of one guiding stitch for correct placement of the valve to the annulus and the possibility of re-position the device if needed.
The Perceval sutureless valve was CE approved in 2011. It comprises a biological component of bovine pericardium and an elastic Ni-Ti alloy stent made of two rings and nine vertical struts, with the dual task of supporting the valve and holding it in place without any permanent suture. Its elastic properties allow the stent to adapt to the anatomy of the aorta and to follow its movements, relieving the stress on the leaflets. The valve is collapsed with an atraumatic device compression, assuring that the valve leaflets are not affected. Perceval is lowered until the correct position and then self-expands back to its original diameter.
The design of the Edwards Intuity valve, CE approved in 2012, is based on the Perimount valve family. A balloon expandable stainless steel cloth-covered frame is incorporated into the inflow aspect of the valve. The valve is implanted with the aid of a delivery system, which incorporates a balloon catheter used to expand the frame within the left ventricular outflow tract. The expandable frame works in conjunction with the sewing ring to position and stabilize the valve at implant. The system reduces the number of sutures required to secure the valve, while establishing the seal between the aortic annulus and the frame.
Current evidence
Current evidence on SU-AVR is limited to observational studies with short-term to mid-term follow-up. In the largest institutional study comparing 164 minithoracotomy versus 117 ministernotomy SU-AVR patients (19), it was found that in-hospital mortality (0.7%), strokes (1.8%) and overall survival rate (90%) over one-year follow-up was acceptable and safe. Cardiopulmonary bypass (81 min) and cross-clamp (48 min) durations were low and excellent mean postoperative gradients were achieved. In the largest multi-center study on sutureless valves (20), analysis of 314 patients showed acceptable early survival in high-risk patients and low paravalvular leak rates (0.6%).
Despite the above retrospective and prospective analyses, there is still a paucity of randomized controlled trials on SU-AVR. Existing propensity-matched studies are insufficiently powered to determine long-term survival outcomes and to adequately compare between different minimally invasive interventions. In a propensity-matched study by D’Onofrio et al. (21), 38 matched pairs of SU-AVR versus TAVI showed that both approaches were equally efficacious, but SU-AVR was associated lower incidence of paravalvular leak and similar transprosthetic gradients. In a similar study by Santarpino et al. (22), SU-AVR demonstrated a significantly higher survival rate than the TAVI group, lower paravalvular leak incidence, shorter procedural durations and non-significant increase in permanent pacemaker implantations. In another study, propensity-matched analysis of 164 pairs (23) receiving sutureless and conventional sutured valves demonstrated reduced procedural time in the SU-AVR cohort, that significantly correlated with shorter hospitalization, reduced postoperative atrial fibrillation, respiratory complications and hospital costs. Most recently, a randomized multicenter trial demonstrated that minimally invasive sutureless approaches was superior to conventional full sternotomy AVR with significantly shortened myocardial ischemic time and better valvular hemodynamic function (24). The authors suggested that sutureless valves may facilitate the performance of minimally invasive AVR.
In order to best assess the current evidence base, the IVSSG recently performed the first meta-analysis of SU-AVR (17), pooling results from 1,037 patients undergoing sutureless aortic valve surgery in 12 eligible studies. Without the need to place and tie sutures, SU-AVR had a pooled cross-clamp and cardiopulmonary bypass durations of 45 and 73 minutes, respectively, and was further shortened for stand-alone AVR procedures (33 minutes, 57 minutes). Some experienced valvular centers have reported cross-clamp and cardiopulmonary bypass durations as low as 22 and 46 minutes, respectively (16). These operative durations are much shorter compared to the reported durations of isolated conventional AVR, and suggest potential benefits from sutureless technology in different settings: higher risk or elderly patients, complex or time-consuming combined operations, and minimally invasive surgery. Pooled 30-day and 1-year mortality rates were 2.1% and 4.9%, respectively, while the incidences of strokes (1.5%), valve degenerations (0.4%) and paravalvular leaks (PVL) (3.0%) were satisfactory. However, the relative paucity of clinical data on the long-term safety, efficacy and hemodynamic profiles of SU-AVR requires further critical assessment.
Limitations
Despite the apparent wealth of information, these observational studies and meta-analyses are not adequate for addressing our objectives outlined below. The current literature is limited by a number of factors, including (I) low patient numbers and inadequately powered individual studies (II) heterogeneous definitions of clinical variables; (III) confounding factors that are unable to be controlled or adjusted without original data; and (IV) insufficient reporting of postoperative outcomes.
Further clinical evidence for sutureless aortic valve surgery is necessary for the development of high-quality evidence based clinical guidelines for SU-AVR. The introduction of multi-institutional databases, propensity-matched analyses from retrospective and prospective registry data will promote closer collaboration among all centers and allow sufficiently powered statistical analyses to provide required robust clinical evidence on the safety, efficacy and hemodynamic outcomes of SU-AVR.
The International Valvular Surgery Study Group (IVSSG)
The IVSSG was established to address the limitations in the current evidence for SU-AVR. The IVSSG comprises a consortium of research centers that are evaluating the current management and outcomes of valvular surgery, with current efforts focused on sutureless aortic valve interventions. It is envisaged that global collaborative efforts will shape clinical guidelines, optimize patient outcomes, and set future directions of research.
IVSSG scientific committee
The Collaborative Research (CORE) Group is the Coordinating Center for IVSSG, and will be responsible for the concept and design, establishing and maintaining the clinical database, performing statistical analyses and coordinating and organizing the research projects. The CORE Group consists of a team of cardiothoracic surgeons, systematic researchers, research fellows, and biostatisticians from Australasia, North America, Asia and Europe. Further information about the CORE Group can be accessed at www.coregroupinternational.org.
CORE Group researchers will be responsible for storing and maintaining the registry. Encrypted data is stored securely and backed-up in three separate locations. Full access to the registry data will be directly accessible to all members of the Steering Committee. All participating centers are welcome to access the data for scientific reports and publications.
IVSSG Research Steering Committee
The development and direction of Sutureless Study Projects will be overseen by IVSSG Research Steering Committee, which is currently composed of 17 academic surgeons from internationally recognized sutureless surgery centers in eight countries. This expert panel from 14 international centers are representative of high-volume minimally invasive aortic valve surgery and sutureless aortic valve centers across the world.
The Steering Committee will be responsible for monitoring the progress of the projecting, including data quality and control and advice on collection and interpretation of the data. The Steering Committee will also ensure that scientific priorities will be addressed in data analysis and scientific quality. The governance of the Steering Committee will be guided by consensus, defined a priori as more than 50% agreement amongst the panel of experts.
IVSSG mission
To overcome the current limitations of the available literature, a multi-center database for operations involving SU-AVR is required to better assess overall safety, efficacy and hemodynamic outcomes. The collaborative pooling of data will identify optimal operative strategies and prognostic factors. In addition, it will generate hypotheses for future research.
Objectives
The primary objective of this project will be to: (I) generate an international multi-center retrospective/prospective database for patients undergoing AVR with a sutureless valve prosthesis; and (II) assess hemodynamic profiles and safety and efficacy short and long-term outcomes of SU-AVR.
The secondary objectives of this project will be to: (I) perform subgroup comparative analyses based on risk-stratified patients, surgical approach (ministernotomy vs. minithoracotomy vs. full sternotomy), associated procedures (including CABG, double valve surgery, etc.); (II) identify potential significant prognostic risk factors for patients undergoing SU-AVR; and (III) expand the electronic archive interdisciplinary.
Establishment of Sutureless Aortic Valve Replacement International Registry (SU-AVR-IR)
The expected duration of the observational electronic database is six years, but may be renewed and extended. This project comprises of two phases: a Retrospective phase and a Prospective phase.
Retrospective database
Participating centers
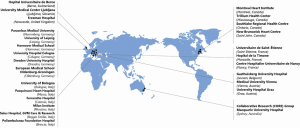
Institutions that have performed greater than 50 SU-AVR operations have been contacted and invited to submit their prospectively collected data. Expert advice was sought from the Research Steering Committee regarding recruitment of other centres. Following this, centers were chosen based on the literature search of international minimally invasive cardiac surgery expertise hubs with consistent and ongoing academic publication output. Currently, 26 tertiary institutions are partaking in this international project and are representative of expert minimally invasive aortic valve surgery and high-volume SU-AVR centers across the world (Figure 2, Table S1). This should minimize bias in patient demographics and surgical skills, thus enabling broader acceptance and application of results. All participating centres are eligible to apply through the Research Steering Committee to perform research projects from the multi-institutional database. All available data from participating centers will be collected, and it is expected that the defined study period will be from 2015-2021 (Figure 3).
Full table
Patients
Ethics approval is obtained from participating institutions through their institutional review boards or through the chairperson of the ethics committee, who waived the need for patient consent for the study as individual patients are not identified. The study population is defined as patients undergoing SU-AVR intervention, either by conventional or minimally invasive incision. Minimally invasive techniques include ministernotomy and minithoracotomy. Sutureless aortic valve types include Perceval S, Intuity and Enable 3F. Patients younger than 18 years will be excluded.
Data collection
An anonymous standard data form has been created to retrieve relevant information. SU-AVR-IR investigators will be asked to submit all institutional retrospective data relevant to SU-AVR performed from 1st December 2010 to 31st December 2014. The SU-AVR-IR retrospective minimum dataset necessary to recruit patients has been defined by the Steering Committee and is in place to ensure validity and enhance quality of data used. Researchers from CORE GROUP International will collate and compile datasets into a homogenised central database, following which researchers from participating institutions can request access to analyzed results.
Prospective database
Participating centers
Institutions participating in the Retrospective Registry will be invited to participate in the Prospective Registry, which is a multi-center, multinational registry with an all-comer design.
Patients
Informed consent for all patients recruited in the prospective phase of the electronic database will be obtained after communicating the objectives and proposes of the study and prior surgery involving the replacement of the aortic valve using a sutureless valve prosthesis. All consecutive adult patients at participating centers who undergo SU-AVR will be recruited from 2015 to 2018, with expected patient follow-up until 2021 (Figure 3). Results obtained from the international retrospective database will be used as a platform to launch the prospective registry, which will allow for continued long-term evaluation of SU-AVR surgical practice evolves with time.
Recruitment will be non-competitive, with each center expected to enroll a pre-assigned number of patients per year according to their real world volume. Participating center do not have to alter their surgical practice.
Clinical data collection
A secured electronic database will be created to allow patient data to be entered by each participating center on a prospective basis. Data fields to be completed represent clinical variables that have significant prognostic values or have clinical implications for future management Definitions and format of the reported data will be homogenized according to the IVSSG Sutureless Projects Variables List. The latter is based on the VARC and VARC-II Consensus Statement for transcatheter AVR procedures and adapted for our surgical aortic valve interventions and long-term clinical and hemodynamic assessment (25,26). Thus, all centers will provide data by using the same definitions and assessment measures, so as to improve the validity of the result. It is hoped that VARC and VARC-II standardized definitions of clinical, echocardiographic and quality of life endpoints reflecting device, procedure and patient-related effectiveness and safety for TAVI may improve and streamline the outcome reporting of SU-AVR as well, and facilitate future comparative registry and prospective randomized studies (25,26). If SU-AVR-IR reveals additional endpoints of clinical significance specific for sutureless valves, the clinical endpoints list can be adjusted for accordingly. Mid-term data with emphasis on SU-AVR efficacy, complications, and hemodynamic outcomes on follow-up will be collected during regular post-operative control visits. Variables of Interest for the SU-AVR-IR include (I) clinical data (including age, sex, NYHA class, CCS class, preoperative AF status, diabetes, indications for surgery, baseline echocardiographic data, previous cardiac or aortic valve surgery, and baseline comorbidities); (II) risk assessment variables (including Logistic EuroSCORE, STS PROM risk, frailty score, and major organ system compromises); (III) operational parameters (including surgical approach (full sternotomy, ministernotomy or minithoracotomy), concomitant procedures such as CABG, MV repair/replacement, number of distal anastomoses, brand of prostheses, prostheses size, cardiopulmonary bypass and cross-clamp duration); (IV) perioperative outcomes (mortality and cause of death, echocardiography parameters, perioperative blood transfusion, renal failure, respiratory failure, myocardial infarctions, conductance disturbance and arrhythmias, cardiac tamponade, endocarditis, valve-related complications, postoperative complications (return to theatre, prolonged ICU stay); duration of ICU stay and duration of hospital stay); and (V) Long-term outcomes (including survival, cardiac and aortic valve re-interventions, as well as clinical and echocardiography outcomes at follow-up). These outcomes have also been previously used by other prospective, multi-center studies investigating sutureless technology (27-30).
Hemodynamic data collection: echocardiography core laboratory
To maintain high quality standards of collected prospective data, the IVSSG is currently considering the use of the echocardiography core laboratory (Echo Core Lab). As a central hub with state-of-the-art echocardiography facilities, the Echo Core Lab will be instrumental in providing comprehensive and accurate assessment of valve hemodynamic performance. The Echo Core Lab has proven to be integral to recent large multi-centric valve registries including Intuity (27,28), 3F-Enable (31), and TAVI (32), allowing validation of echocardiography data, multiparametric approaches and specialist interpretations of valve-related complications.
Statistical analysis
Statistical analysis will be conducted on an as-treated basis. Continuous variables with normal distribution will be expressed as means and standard deviations. Discrete variables were expressed as frequencies and percentages. Statistical comparison of baseline characteristics and outcomes was performed using the Chi-square test or Fisher’s exact test, when appropriate, for categorical variables and the Student’s t-test or the Mann-Whitney test for continuous variables. To compensate for the intrinsic heterogeneity between patient cohorts, propensity-scored matching analysis will be used to evaluate genuine effects of surgical variations on clinical outcomes. Following propensity score matching, subgroup comparisons will be conducted. Event-free survival is calculated by Kaplan-Meier methods with 95% confidence interval and compared with log-rank test.
Conclusions
SU-AVR is an innovative, new approach which continues to be developed and shaped by ongoing technological advances. Given its recent introduction to the field of valvular surgery, the evidence outlining its safety, efficacy, hemodynamic profile and potential complications is predominantly limited to small-volume observational studies and occasional comparative publications, with one RCT performed to date.
The current lack of robust clinical evidence for sutureless aortic valve surgery prevents the development of high-quality evidence based clinical guidelines for SU-AVR. The introduction of multi-institutional databases, appropriate analyses from retrospective and prospective registry data will promote closer collaboration among all centers and allow sufficiently powered statistical analyses for risk factor prediction and indications for SU-AVR surgery based on patient risk profiles and predicted prognosis.
Data and statistical analyses from the retrospective and prospective international registries will provide the basis for scientific publications on short- and long-term efficacy, complications and hemodynamic outcomes of SU-AVR, as well as potential risk factors and prognosis.
The SU-AVR-IR initiated by the IVSSG will be the first independent global collaborative effort with the aim of providing the best evidence available for SU-AVR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002-12. [PubMed]
- Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. Eur Heart J 2010;31:416-23. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5.
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [PubMed]
- Phan K, Zhou JJ, Niranjan N, et al. Minimally invasive reoperative aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:15-25. [PubMed]
- Phan K, Xie A, Tsai YC, et al. Ministernotomy or minithoracotomy for minimally invasive aortic valve replacement: a Bayesian network meta-analysis. Ann Cardiothorac Surg 2015;4:3-14. [PubMed]
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714-20. [PubMed]
- Kapadia SR, Tuzcu EM, Makkar RR, et al. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation 2014;130:1483-92. [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [PubMed]
- Gotzmann M, Bojara W, Lindstaedt M, et al. One-year results of transcatheter aortic valve implantation in severe symptomatic aortic valve stenosis. Am J Cardiol 2011;107:1687-92. [PubMed]
- Généreux P, Webb JG, Svensson LG, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol 2012;60:1043-52. [PubMed]
- Eggebrecht H, Schmermund A, Voigtländer T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129-38. [PubMed]
- Flameng W, Herregods MC, Hermans H, et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg 2011;142:1453-7. [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11.
- Ranucci M, Frigiola A, Menicanti L, et al. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis 2012;21:732-9. [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [PubMed]
- Rubino AS, Santarpino G, De Praetere H, et al. Early and intermediate outcome after aortic valve replacement with a sutureless bioprosthesis: Results of a multicenter study. J Thorac Cardiovasc Surg 2014;148:865-71; discussion 871. [PubMed]
- D'Onofrio A, Messina A, Lorusso R, et al. Sutureless aortic valve replacement as an alternative treatment for patients belonging to the "gray zone" between transcatheter aortic valve implantation and conventional surgery: a propensity-matched, multicenter analysis. J Thorac Cardiovasc Surg 2012;144:1010-6. [PubMed]
- Santarpino G, Pfeiffer S, Jessl J, et al. Sutureless replacement versus transcatheter valve implantation in aortic valve stenosis: a propensity-matched analysis of 2 strategies in high-risk patients. J Thorac Cardiovasc Surg 2014;147:561-7. [PubMed]
- Pollari F, Santarpino G, Dell'Aquila AM, et al. Better short-term outcome by using sutureless valves: a propensity-matched score analysis. Ann Thorac Surg 2014;98:611-6; discussion 616-7. [PubMed]
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013;145:6-23. [PubMed]
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205-17. [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [PubMed]
- Haverich A, Wahlers TC, Borger MA, et al. Three-year hemodynamic performance, left ventricular mass regression, and prosthetic-patient mismatch after rapid deployment aortic valve replacement in 287 patients. J Thorac Cardiovasc Surg 2014;148:2854-60. [PubMed]
- Shrestha M, Folliguet T, Meuris B, et al. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis 2009;18:698-702. [PubMed]
- Shrestha M, Folliguet TA, Pfeiffer S, et al. Aortic valve replacement and concomitant procedures with the Perceval valve: results of European trials. Ann Thorac Surg 2014;98:1294-300. [PubMed]
- Englberger L, Carrel TP, Doss M, et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014;148:1681-7. [PubMed]
- Rodés-Cabau J, Webb JG, Cheung A, et al. Long-term outcomes after transcatheter aortic valve implantation: insights on prognostic factors and valve durability from the Canadian multicenter experience. J Am Coll Cardiol 2012;60:1864-75. [PubMed]






