Current state of transcatheter mitral valve repair with the MitraClip
Introduction
Mitral valve disease is relatively common, affecting approximately 1.7% of the United States population (1). Several techniques have been developed for surgical correction of clinically significant moderate or severe mitral valve disease, but until the past decade, patients who were at extreme surgical risk or inoperable had no options beyond medical therapy. The MitraClip device (Abbott Vascular, Menlo Park, CA, USA) has significantly broadened the treatment options for patients with mitral regurgitation (MR). This device was developed from the concept of the surgical edge-to-edge leaflet repair technique, pioneered in the 1990s by Alfieri and adapted as a percutaneous therapy (2). Success of the MitraClip program depends on a comprehensive valve team approach to ensure thorough evaluation of the valve disorder and co-morbidities, followed by selection of the most appropriate therapy for each patient.
The device is currently approved in the United States for patients with symptomatic primary (degenerative) MR who are at prohibitive risk for surgery, and remains under study to determine its efficacy in functional MR [via the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial (3)]. Our objectives are to: (I) describe the patient selection process and procedural techniques; (II) describe our institution’s early results and outcomes from MitraClip repair in high-risk patients; and (III) discuss the current state of MitraClip technology and the role of cardiothoracic surgeons in a MitraClip program.
Methods
Patient selection
All patients referred to our center for consideration of MitraClip therapy were evaluated by an experienced mitral surgeon and a structural heart interventional cardiologist. Initial evaluation included: (I) transthoracic echocardiography to assess severity of MR, mitral valve area, and concomitant structural disease; (II) transesophageal echocardiography (TEE) to characterize valve pathology, and anatomic feasibility of MitraClip. Several additional studies are needed in many cases to assess surgical risk, including: (I) chest computed tomography to assess calcium burden of ascending aorta and degree of mitral annular calcification; (II) right and left heart catheterization to assess pulmonary vascular resistance and coronary artery disease; and (III) pulmonary function testing. Specific requirements for MitraClip placement include a resting effective orifice area greater than 4 cm2 to limit the risk of post-procedural stenosis, a single dominant regurgitant jet, and leaflet edges free of calcification. Each case was discussed by a multidisciplinary valve team composed of surgeons, interventional cardiologists, nurse practitioners, imaging specialists, as well as pulmonologists, nephrologists, oncologists, geriatricians, and hepatologists as needed, to gauge the optimal treatment for each patient. Our group has been involved in MitraClip trials and registries for nearly ten years, and continues to carefully evaluate each patient’s risk profile and suitability for the procedure, whether for a research study or commercial MitraClip use. Patients with symptomatic, degenerative MR 3+ or greater who are at prohibitive risk for surgery are candidates for commercial MitraClip (4). Patients with functional MR and symptomatic heart failure who are deemed unsuitable for surgery could potentially be enrolled in the COAPT trial with randomization to optimal medical management or medical management plus MitraClip (5).
Operative procedure
The MitraClip procedure is performed in a hybrid operating suite due to the increased space requirements for anesthesia and TEE teams. General anesthesia is used to protect the airway during TEE. Once percutaneous venous access is accomplished, hemodynamic right heart measurements are performed, and then transseptal puncture is made through the atrial septum, guided by TEE. The goal of this method is to secure a transseptal position that is relatively posterior on the septum, and 4 cm above the point of pathology on the mitral valve. Following the initial transseptal puncture, a 0.032 wire is passed into the left atrium. After confirming an appropriate transseptal location with the wire passing, the patient is heparinized, and the atrial septum is dilated. Next, the femoral venous access tract is dilated with an 18 French dilator, and then the 24 French steerable MitraClip guide is passed into the left atrium. Using fluoroscopic and echocardiographic guidance, the MitraClip is driven through the left atrium and positioned over the pathology on the mitral valve with the MitraClip arms perpendicular to the line of coaptation of the valve leaflets. The MitraClip is then passed across the valve and gradually pulled back to the ventricular side of the valve with the clip arms at 120°. Once the clip position is optimized by TEE, the frictional grippers are dropped and the clip arms are closed. If the clip is not in optimal position, it can be released and repositioned prior to release. If significant MR remains and no evidence of mitral stenosis is detected, additional clips may be placed at the area of greatest remnant regurgitation. The guidance system is removed, protamine is given, and femoral venous sheath is removed when the activated clotting time (ACT) has normalized. The femoral access is closed with a silk pursestring in the skin. Antiplatelet therapy is instituted after the procedure, and patients are observed for 24-48 hours prior to discharge.
Study design
We reviewed early outcomes data for 115 high-risk patients treated with MitraClip at our institution. Patient demographic information, preoperative evaluation, intraoperative details, and postoperative course data were collected from our valve center database as well as review of the electronic medical record system. We sought to evaluate our entire patient cohort at our institution from the REALISM continued-access registry through the early commercial MitraClip experience.
Outcomes
The primary outcome of interest was 30-day mortality from the MitraClip procedure. Secondary outcomes included degree of reduction of MR, overall improvement in New York Heart Association (NYHA) class, length of stay, and major complications (such as the need for transfusion, stroke, new-onset atrial fibrillation and myocardial infarction).
Results
UVA results: case example
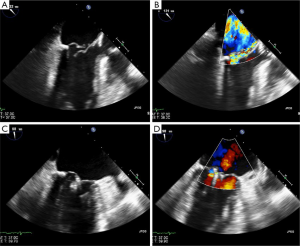
An 88-year-old man was referred to our institution for consideration of coronary artery bypass grafting and mitral valve repair/replacement, versus complex percutaneous coronary intervention and MitraClip placement. Given his advanced age, frailty, as well as severe pulmonary hypertension (pulmonary artery systolic pressure of 110 mmHg), he was deemed unfit for surgery. TEE demonstrated posterior leaflet prolapse and ruptured chordae with severe, eccentric regurgitation (see Figure 1). He underwent left anterior descending coronary and right coronary artery stenting followed by MitraClip placement one day later with reduction of MR from 4+ to 2+. At one month follow-up he described complete symptomatic relief and was able to exercise on a treadmill without issue.
UVA results: early MitraClip outcomes
Outcomes
The patients treated at our institution demonstrated similar characteristics to those involved in the broader clinical trials and registries. Average Society for Thoracic Surgeons (STS) mortality risk was 9.4%. More than 40% had a history of previous cardiac surgery. Medical comorbidities, including previous myocardial infarction (in 40.9%), were common (see Table 1).
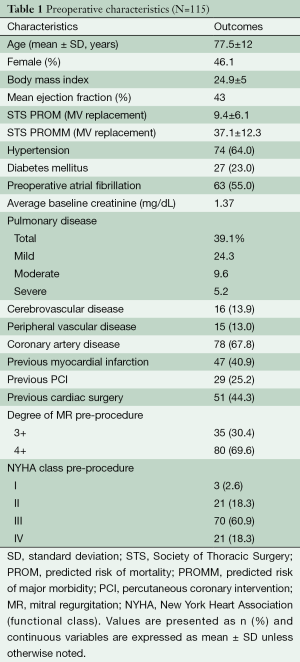
Full table
Of 115 patients, 114 (99.1%) patients had successful MitraClip placement with one aborted procedure due to inability to place the clip in a patient with a dehisced mitral annuloplasty ring obstructing the mitral inflow. The 30-day mortality was 2.6% for the group including two patients in hospital and one at home two weeks after discharge. MR was reduced from 3+ to 4+ pre-procedure to trace or 1+ in 77 patients (86%), 2+ in 15 patients (13.2%), and 3+ in one patient (0.9%). None of the patients who underwent clip placement required mitral valve surgery in the 30 days after clip. From baseline to 1 month post-clip, MR decreased, on average, by 2.15 grades. Most (79%) patients were characterized as NYHA symptom class III or IV preoperatively; at 1 month post-clip placement, 81% of patients exhibited NYHA symptom class I or II. Average length of hospital stay was 2.8 days. Two patients (1.7%) experienced post-procedural stroke, one with full recovery. Three patients (2.6%) experienced new-onset atrial fibrillation and no patients required transfusion of more than two units or had a periprocedural myocardial infarction (see Table 2).
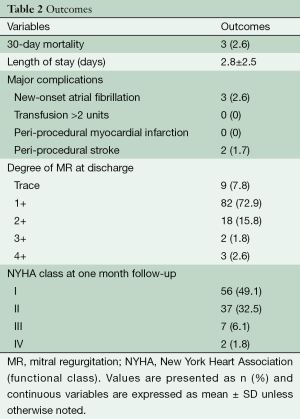
Full table
Discussion
Review of the literature
EVEREST trials
The initial MitraClip feasibility study, EVEREST I, included 27 patients with 3+ to 4+ MR who could potentially undergo surgery should complications arise during or after clip placement. Of this initial group, 64% of patients discharged with a clip in place had 2+ MR or less at one month, and remained stable at six months. Six of the 27 patients went on to have surgery, either due to failed clip placement, partial clip detachment, or lack of improvement in MR. This initial study demonstrated the benefits of percutaneous valve repair for a large proportion of patients, and also illustrated the learning curve associated with the procedure and its requirement for well-coordinated efforts of the interventionalists and echocardiographers to ensure procedural success (6).
The EVEREST II trial included a randomized controlled study of MitraClip versus surgical mitral valve repair or replacement, whereby 184 patients underwent clip placement, while 95 underwent surgery. The study continued to demonstrate safety of the device; ten patients experienced detachment from a single leaflet but no clip embolizations were observed, and only one patient experienced late development of mitral stenosis (four years after initial clip implant) as a result of the clip procedure. There was no significant difference between the clip and surgery groups in overall rate of death or 3+ or 4+ MR at four years. The clip group did require significantly more surgical intervention within four years of the index procedure, at 24.8%, compared to only 5.5% of the surgical group requiring repeat operation. The majority of re-interventions in the clip group occurred within one year of clip placement, while the need for repeat surgery after initial surgical procedure was distributed over the five year follow-up period (7).
REALISM data
The EVEREST II high-risk registry and REALISM Continued Access Study High-Risk arm collected data for patients at high risk for surgery treated with MitraClip. Patients had an STS surgical mortality risk of 12% or greater (or STS risk <12% or at least one protocol-defined risk factor), and symptomatic 3+ or 4+ MR. The 12-month results of these registries were recently published, with 327 of 351 patients completing 12 months of follow-up. In this high-risk group, 30-day mortality of 4.8% was observed with no deaths related to device failure. While 16.4% of patients had MR >2+ at one year, the rate of surgery was low at 2.2%, consistent with the patients’ high surgical risk at enrollment. Overall, patients experienced significant improvements in MR grade, NYHA class, quality of life scores, and left ventricular dimensions (8).
To further clarify the outcomes of patients with degenerative MR at prohibitive risk for surgery, a group of high-risk patients from the pooled EVEREST II high-risk arm and the REALISM continued access registry was studied and prohibitive surgical risk patients were identified. For the 127 patients in this group, a 95% initial success rate was achieved. 30-day mortality was 6.3%, with an average STS score of 13.2%±7.3%. At one year post-clip, a mortality rate of 23.6% was observed, reflecting the patients’ baseline comorbidities, and 57.5% demonstrated NYHA functional status of I or II at one year post-clip compared with only 12.6% with NYHA I or II at baseline. The clear benefits observed in patients who otherwise were at prohibitive surgical risk further underscored the pertinence of a certified clinical application of the MitraClip in the United States (9).
Commercial approval
The results of the EVEREST trials and REALISM registry demonstrated the overall safety and efficacy of MitraClip for patients at high or prohibitive risk for surgery. This has led to the recent Food and Drug Administration (FDA) approval of the MitraClip for use in patients with symptomatic, degenerative MR grade 3+ or 4+ who are at high risk for surgery.
Heart team approach
The success of MitraClip depends heavily on a comprehensive heart team approach. Imaging experts are required to accurately characterize the mechanism of MR. Cardiologists, including heart failure specialists, are integral to the patient evaluation process, as it is often difficult to determine whether a given patient’s symptomatology can be attributed to MR versus ventricular dysfunction or lung disease. With this type of team approach and significant experience through the trajectory of MitraClip development, we have demonstrated continuing improvements in MR reduction and mortality over time. Our group of 115 high-risk patients, with a mean STS mortality risk score of 9.4%, had a 30-day mortality rate of only 2.6%, an improvement over the reported high-risk registries and reflecting the benefits of a comprehensive team approach.
Role of surgeons
As with other transcatheter therapies, concerns have arisen that the MitraClip procedure may detract from surgical volumes. Conversely, by having surgeons as an integral part of patient evaluation and as ‘gatekeepers’ seeing patients early in the process to gauge suitability for surgery, we have found that our institution’s mitral surgery volumes have increased. Additionally, surgeons can be involved in the MitraClip procedure itself; at our center, surgeons have performed a significant percentage of cases since 2011. Our experience represents the first surgeon involvement in the MitraClip procedure in the United States (10). The MitraClip technology certainly offers patients an alternative treatment without the invasiveness of surgery, and surgeons continue to provide essential input in the process of patient selection, procedural performance and long-term follow-up.
Conclusions
The MitraClip procedure now provides a safe and effective alternative for many patients with MR who are deemed unfit for surgery. Outcomes have continued to improve with greater experience, with better reduction in MR and less morbidity and mortality. Surgeons can and should have an active role in the selection of appropriate patients as well as performance of the procedure.
Acknowledgements
We would like to thank Kristin Miller, our excellent Advanced Cardiac Valve Center clinic coordinator, for her assistance with patient records and data collection.
Disclosure: The authors declare no conflict of interest.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [PubMed]
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-81. [PubMed]
- COAPT Clinical Trial, ClinicalTrials.gov [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT01626079
- FDA approval MitraClip. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf10/p100009a.pdf
- Cardiovascular Outcomes Assessment of the MitraClip Therapy Percutaneous Therapy for High Surgical Risk Patients - Full Text View - ClinicalTrials.gov. Available online: http://clinicaltrials.gov/show/NCT01626079
- Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol 2005;46:2134-40. [PubMed]
- Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013;62:317-28. [PubMed]
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [PubMed]
- Lim DS, Reynolds MR, Feldman T, et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J Am Coll Cardiol 2014;64:182-92. [PubMed]
- Ailawadi G, Lim D, Mack M, et al. Initial North American Experience of MitraClip Procedure Performed by Cardiac Surgeons. AATS Mitral Conclave, New York, NY, 2011. Available online: http://www.aats.org/mitral/abstracts/2011/50.cgi





