Repair or observe moderate ischemic mitral regurgitation during coronary artery bypass grafting? Prospective randomized multicenter data
Introduction
Mitral regurgitation (MR) is the most common heart valve disease in the United States, affecting approximately 1.7% of the American population (1-3). As the US population continues to age, the incidence of MR is expected to rise (1). The presence of MR has repeatedly been associated with adverse cardiac events and mortality (2,4-6). Due to the poor outcomes seen with coronary artery disease associated with MR, cardiac operation is often indicated, especially in symptomatic patients (7). However, there remains considerable debate regarding the most appropriate treatment option.
MR is traditionally classified by both the etiology and the underlying mechanism. Classifications based on etiology include “ischemic” and “non-ischemic”, while classifications based on mechanism include “functional” and “structural” (2). Ischemic MR (IMR) is functional MR that occurs secondary to the effects of coronary artery disease (2). In functional MR, ventricular remodeling results in both annular dilation and lateral displacement of the papillary muscles. This tethers the leaflets of the mitral valve resulting in regurgitation (2). MR is further graded as mild, moderate, or severe based on ventriculography or echocardiography using many differing criteria (2,8), with increasing MR grade demonstrated to be associated with increased mortality and cardiovascular events (6).
Approximately 20% of MR is ischemic and associated with myocardial infarction (MI) (2,9). In a study of 1,331 patients, 50% developed MR within thirty days of an MI (9). While the majority of cases of MR in this study were mild, 12% of patients had either moderate or severe MR. Severity of IMR directly impacts 1-year survival; ranging from 85% for patients with mild IMR to 75% for those with severe IMR (10-12). The presence of MR prior to other cardiac operations has also been associated with worse outcomes, even when only mild in severity (13).
Moderate ischemic mitral regurgitation
While most authors believe that treatment of IMR should vary based on severity, there is substantial disagreement as to the most appropriate treatment for each grade of disease. The subject of debate surrounding therapy for moderate IMR is whether its treatment should be coronary artery bypass grafting (CABG) alone or CABG in conjunction with mitral valve surgery. Proponents of isolated CABG argue that by treating the underlying cause, revascularization leads to reverse remodeling of the left ventricle which in turn decreases the tethering of the mitral valve leaflets and results in a reduction in regurgitation (14). Proponents of combined CABG and mitral valve surgery argue that the success of isolated revascularization depends on the extent of viable myocardium (15). Therefore, sufficient ventricular remodeling may not occur with isolated CABG in the presence of scar, thus necessitating concomitant mitral valve repair (MVR) or replacement. Supporting this argument, Penicka et al. demonstrated that improvement in MR in patients with moderate mitral regurgitation (MMR) who underwent an isolated CABG was limited to patients who had viable myocardium and an absence of dyssynchrony between papillary muscles (15). In addition, Aklog et al. demonstrated that roughly 40% of patients continued to have moderate MR following isolated CABG for MMR (16). Conversely, concomitant mitral surgery has been associated with increased operative mortality when compared to CABG alone (17,18).
This debate has been fueled by the publication of numerous observational and randomized studies, each of which has differed in design, MR definition and study population, resulting in conflicting findings (Tables 1,2). Some of the retrospective studies failed to appropriately discriminate true IMR from other etiologies, while two of the three currently published randomized studies were limited by small sample sizes (19-21). Most of these studies failed to demonstrate either a survival advantage or a symptomatic benefit with concomitant mitral surgery. For example, Wong et al. retrospectively reviewed 251 patients with MMR who underwent CABG (22). In this study, 31 patients underwent a concomitant mitral annuloplasty. Due to its limited sample size, this study failed to demonstrate a survival advantage for mitral annuloplasty at a median follow-up of 4.3 years. However, the group of patients that underwent concurrent MVR did have significantly less MR, at a median follow-up of one year, than the isolated-CABG group (22). Harris et al. similarly investigated 176 patients with MMR, of whom 34 underwent concomitant MVR (18). In this study, operative mortality was significantly higher in the group undergoing valve surgery (21% vs. 9%); but there was no significant difference in recurrent MR, New York Heart Association (NYHA) heart failure class, or overall survival at five years. Interestingly, in a subset of patients with NYHA heart failure class III and IV prior to surgery, concomitant annuloplasty was associated with significantly improved 5-year survival (18).
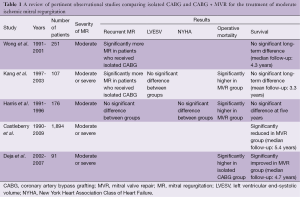
Full table
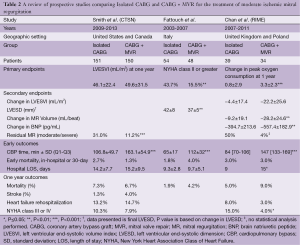
Full table
Corroborating some of the findings of these retrospective studies, the results of the first prospective randomized controlled trial comparing isolated CABG vs. CABG plus MVR in patients with MMR was published in 2009 (20). This single center Italian study randomized 102 patients; 48 patients underwent CABG plus MVR, while 54 underwent CABG alone. The primary endpoints were NYHA functional class, left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and ejection fraction (EF). MR was graded both by transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) (20). In this study, only 15.5% of patients in the CABG plus MVR group demonstrated NYHA class II or greater heart failure symptoms versus 43.7% in the isolated CABG group (P=0.002) at the time of last follow-up (20). Additionally, CABG-only patients had higher rates of post-operative MR, with lesser improvements in LVESD and LVEDD. However, this study failed to demonstrate improvement in EF or survival (20).
Deja et al. performed a sub-analysis of STICH (Surgical Treatment for Ischemic Heart Failure Trial) to investigate the role of concomitant MVR and CABG on long-term outcomes in patients with IMR (23). Ninety-one patients in this trial had moderate to severe MR, 49 of whom underwent MVR in addition to CABG. MVR was associated with significantly longer ICU and hospital stays, but in contrast to previous studies, was also associated with a significantly decreased rate of 30-day mortality (17,18,23). Also, in contrast to previously discussed studies, MVR was associated with significantly improved long-term survival even after adjustment for other patient characteristics (hazard ratio: 0.41; 95% confidence interval: 022-0.77) (23). These results must be interpreted in light of the inclusion criteria of STICH, which only included patients with a decreased EF at baseline (<35%). Despite this, in a study of IMR patients with an LVEF <50%, Goland et al. did not corroborate the results of the STICH subanalysis (24).
Further clouding the picture, the results of the Randomized Ischemic Mitral Evaluation (RIME) trial were published in 2012 (21). This multicenter prospective trial was performed in the United Kingdom and Poland. The trial only randomized 73 out of a planned total of 100 patients who had moderate MR by echocardiography to either isolated CABG or concomitant CABG plus MVR, as the study was stopped early after the primary endpoint reached significance. The primary endpoint was the peak oxygen consumption at one year. Secondary endpoints included left ventricular end systolic volume index (LVESVI), MR volume, and brain natriuretic peptide (BNP) levels, all at one year (21).
In the RIME trial, CABG plus MVR (n=34) patients had a 22% increase in their peak oxygen consumption at one-year compared to only a 5% increase in the isolated CABG group (P<0.001). Additionally, CABG plus MVR patients had a 28% decrease in LVESVI compared to a 6% decrease in the isolated CABG group (P=0.002), as well as lower MR grade and BNP levels. Lastly, the CABG plus MVR group had a median NYHA functional class of I compared to II in the isolated CABG group. Despite this, no difference in survival was identified and there was a trend towards increased post-operative complications in the CABG plus MVR group including dialysis requirement, re-operation for bleeding or tamponade, and stroke (21).
Although limited by small sample size and limited follow-up, the RIME trial provides evidence to support the use of MVR in this patient population (21). The primary endpoint, peak oxygen consumption, is clinically relevant having been recognized as a measure of functional capacity, and demonstrated to have a strong association with survival (25-27). Detractors of this study argue that isolated CABG relies on reverse ventricular remodeling in order to treat MR, a process that can take longer than a year (28). Alternatively, MVR results in an immediate resolution of MR. Therefore, comparison of MR rates at one year may be unjustly biased in favor of MVR. As a result, the differences in peak oxygen consumption in this study may disappear at longer follow-up.
The largest prospective randomized trial to date is the Surgical Treatment of Moderate Ischemic Mitral Regurgitation Trial, conducted by the Cardiothoracic Surgical Trials Network (CTSN) (29). This trial randomized 301 patients at 26 sites to CABG plus MVR using an undersized complete ring (n=150) or isolated CABG (n=151). The primary end point was left ventricular end-systolic volume index (LVESVI) at 12 months and secondary endpoints included a major adverse cardiac or cerebrovascular events (MACCE) composite, as well as mortality, functional status, and quality of life (29,30).
In an effort to better approximate clinical practice, patients were enrolled in this study without a “run-in” period that would have allowed for optimization of their medical therapy prior to their qualifying TTE (30). Following their qualifying imaging, where moderate MR was defined using integrative criteria by a certified site echocardiographer, patients remained in the trial regardless of subsequent MR grade change prior to surgery (8,30). Furthermore, since several studies have demonstrated that the severity of MR may change with anesthesia and loading conditions, the intraoperative transesophageal echocardiograph was utilized solely as a final determination of structural normality of the mitral apparatus (31). This theoretically led to the inclusion of patients with more “real world” MR for analysis (30).
Both arms of the study had improved LVESVI (LV remodeling) at one year (29). Unlike the RIME trial, there was no significant difference in LVESVI between the groups (21,29). Similar to other studies, isolated CABG patients had more residual MR (31.0% vs. 11.2%, P<0.001), but this was not associated with any difference in MACCE or survival at 12 months (16,22,29). Also in contrast to previous studies, there was no difference in 30-day mortality, functional status, or quality of life at 1 year between the two groups (17,18,20,21,29).
The CTSN study did demonstrate a significantly higher rate of neurologic events (i.e., stroke, transient ischemic attack, and metabolic encephalopathy) as well as supraventricular arrhythmias in the CABG plus MVR group as compared to the CABG alone group (29). Both of these findings may be secondary to the left atriotomy required for the MVR. Patients in the CABG plus MVR group had significantly longer operative times, as well as ICU stays, but this did not translate to different rates of readmission between groups (29).
The differing results of the RIME and the CTSN trials may, in part, be explained by their study design and the baseline differences in the study population. The RIME population was older, more often male, and excluded patients with NYHA class IV heart failure (21). In the CTSN trial, the cardiopulmonary bypass and aortic cross-clamping times were substantially longer, implying that the CTSN surgeons may have used different operative techniques (29). Ultimately however, the CTSN study had a more rigorous methodology. It required that the operating surgeons perform, on average, a minimum of ten MVRs a year; all patients had to have a transesophageal echocardiogram to rule out any structural valve abnormality; and all echocardiograms were interpreted by a blinded core laboratory (29,30).
The largest study to date regarding the treatment of moderate and severe IMR is by Castleberry and colleagues (32). This group examined 4,989 patients with IMR over a 10-year period at a single center. Patients in this study either received medical management (n=1,800), revascularization with PCI (n=1,295), surgical revascularization with CABG (n=1,651), or concomitant CABG and MVR (n=243). In this large study, isolated CABG achieved the highest adjusted survival at 10 years, with a median follow-up of 5.37 years (Figure 1). Additionally, there was no significant association between severity of MR and overall survival regardless of treatment type (32). Although this publication is retrospective, it represents the largest and most comprehensive study of survival after mitral intervention to date.
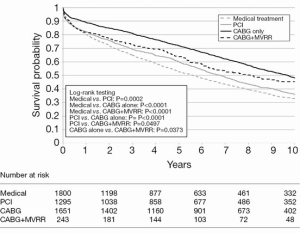
Conclusions and future directions
Despite contradictory smaller studies, the largest studies regarding MMR demonstrate no survival benefit associated with CABG and concomitant MVR at one year (29). While it is likely that there is a subset of patients whose survival, functional status, or symptoms may improve with MVR, this subset of patients has yet to be definitively identified. Soon, the CTSN trial will report 2-year survival outcomes and thereby provide more evidence regarding the long-term impact of revascularization in IMR. Furthermore, this trial gathered specific data on neurologic function, functional status, cardiac wall motion, and detailed operative techniques (30). Planned subgroup analyses of the CTSN trial will be critical to our understanding of IMR and its treatment. In particular, the planned analysis of sub-segmental wall motion based on TEE and its impact on IMR will be paramount.
In conclusion, the best clinical data available today supports isolated CABG as first line therapy in the short term for moderate ischemic MR. Ongoing follow up and subgroup analyses of current data may identify groups of moderate IMR patients that would most benefit from concomitant MVR and CABG.
Acknowledgements
Funding: Supported by a cooperative agreement (5UM1HL088953-07) with the National Heart, Lung, and Blood Institute.
Disclosure: The authors declare no conflict of interest.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [PubMed]
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [PubMed]
- Little SH, Igo SR, Pirat B, et al. In vitro validation of real-time three-dimensional color Doppler echocardiography for direct measurement of proximal isovelocity surface area in mitral regurgitation. Am J Cardiol 2007;99:1440-7. [PubMed]
- Avierinos JF, Gersh BJ, Melton LJ 3rd, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002;106:1355-61. [PubMed]
- Pellizzon GG, Grines CL, Cox DA, et al. Importance of mitral regurgitation inpatients undergoing percutaneous coronary intervention for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol 2004;43:1368-74. [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [PubMed]
- Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. [PubMed]
- Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759-64. [PubMed]
- Feinberg MS, Schwammenthal E, Shlizerman L, et al. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol 2000;86:903-7. [PubMed]
- Trichon BH, Felker GM, Shaw LK, et al. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003;91:538-43. [PubMed]
- Schroder JN, Williams ML, Hata JA, et al. Impact of mitral valve regurgitation evaluated by intraoperative transesophageal echocardiography on long-term outcomes after coronary artery bypass grafting. Circulation 2005;112:I293-8. [PubMed]
- Foster E, Kwan D, Feldman T, et al. Percutaneous mitral valve repair in the initial EVEREST cohort: evidence of reverse left ventricular remodeling. Circ Cardiovasc Imaging 2013;6:522-30. [PubMed]
- Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation 2009;120:1474-81. [PubMed]
- Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation 2001;104:I68-75. [PubMed]
- Kang DH, Kim MJ, Kang SJ, et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation 2006;114:I499-503. [PubMed]
- Harris KM, Sundt TM 3rd, Aeppli D, et al. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thorac Surg 2002;74:1468-75. [PubMed]
- Filsoufi F, Salzberg SP, Adams DH. Current management of ischemic mitral regurgitation. Mt Sinai J Med 2005;72:105-15. [PubMed]
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg 2009;138:278-85. [PubMed]
- Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [PubMed]
- Wong DR, Agnihotri AK, Hung JW, et al. Long-term survival after surgical revascularization for moderate ischemic mitral regurgitation. Ann Thorac Surg 2005;80:570-7. [PubMed]
- Deja MA, Grayburn PA, Sun B, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation 2012;125:2639-48. [PubMed]
- Goland S, Czer LS, Siegel RJ, et al. Coronary revascularization alone or with mitral valve repair: outcomes in patients with moderate ischemic mitral regurgitation. Tex Heart Inst J 2009;36:416-24. [PubMed]
- Myers J, Gullestad L, Vagelos R, et al. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann Intern Med 1998;129:286-93. [PubMed]
- Francis DP, Shamim W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 2000;21:154-61. [PubMed]
- Arena R, Myers J, Williams MA, et al. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007;116:329-43. [PubMed]
- Bax JJ, Braun J, Somer ST, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation 2004;110:II103-8. [PubMed]
- Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2014;371:2178-88. [PubMed]
- Smith PK, Michler RE, Woo YJ, et al. Design, rationale, and initiation of the Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial: a report from the Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg 2012;143:111-7, 117.e1.
- Bach DS, Deeb GM, Bolling SF. Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am J Cardiol 1995;76:508-12. [PubMed]
- Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation 2014;129:2547-56. [PubMed]





