Posterior annulus shortening increases leaflet coaptation in ischemic mitral incompetence: a new and valid technique
Introduction
Ischemic mitral incompetence (IMI) is a complex multifactorial disease related to coronary artery disease, which is a frequent complication of myocardial infarction and is associated with poor prognosis (1). It involves global and regional left ventricular remodeling as well as dysfunction and distortion of the mitral valve (MV), including the chordae, annulus and leaflets. It is characterized by restrictive leaflet mobility due to dyskinesia/akinesia of the ventricular wall, which bears one or both papillary muscles thus extending the distance between the ventricular wall and the leaflets. The posterior papillary muscle with its supporting ventricular wall and the posteroinferior wall of the left ventricle (LV) are most frequently affected. Thus, in IMI, mitral insufficiency may be caused by dilatation of the annular-ventricular apparatus, dysfunction of the LV wall alongside its papillary muscles or a combination of these factors, resulting in failure of leaflet coaptation (2). As the MV structure appears ostensibly normal in IMI, the lesion is ideal for valve repair.
While undersized ring annuloplasty has emerged as the preferred surgical treatment of IMI by being able to reduce the annular size (3), mid-term and long-term results have been disappointing, with several experienced centers reporting significant residual and recurrence rates of moderate to severe mitral incompetence in 30% of patients within 6 months of surgery (4-8). It may even potentiate leaflet tethering, decrease leaflet curvature and result in increased leaflet and chordal stress (9,10). This exacerbation of abnormal leaflet geometry may contribute to the suboptimal repair results for IMI. To improve surgical outcomes, various subvalvular and leaflet techniques have been attempted such as secondary chordal cutting (11), papillary muscle modification (12), posterior leaflet augmentation (13-16) and anterior leaflet augmentation (17). These procedures, however, only have been employed in small number of patients, and long-term results have yet to be fully evaluated.
We introduce a simple and reproducible strategy of a posterior annulus shortening technique which addresses the restrictive mitral leaflet mobility in IMI, and report its long-term outcome.
Methods
The Institutional Review Board approved this study and waived the need for patient consent.
Patients
Between May 1986 and December 2012, we performed a total of 29,503 valve operations, of which 9,742 were MV surgeries (4,570 replacements and 5,172 repairs). Seventy-five patients (mean age, 64.56±10.37 years; median, 66.0 years; range, 35.0-86.1 years) had IMI from either global myocardial ischemia, postinfarction ischemia or transient ischemia. In these situations, MV insufficiency is mostly due to incompetence around the posterior parts of both the anterior and/or posterior leaflets, seen as leaflet tethering and absence of leaflet coaptation related to ischemia of the inferoposterior wall of the LV. This occurs mostly in obstruction of the right coronary artery and/or left circumflex coronary artery. Most patients (83%) presented with triple-vessel disease with a high prevalence of angina pectoris (70%). Eighty percent of patients had impaired LV function [mean left ventricular ejection fraction (LVEF) 0.32], accounting for the high incidence of patients in New York Heart Association (NYHA) functional class III or IV. Patients also had significant non-cardiac co-morbidities, including diabetes mellitus, hypertension, cerebrovascular events, peripheral arterial disease and chronic obstructive pulmonary disease. Forty-nine (65.3%) patients underwent MV repair (posterior annulus shortening technique) with concomitant coronary revascularization and 26 (34.7%) patients underwent isolated MV repair. These patients had previous coronary revascularization with mild MI upon discharge, which progressed in severity over time.
Ruptured chordae are typically found in only a very small proportion of cases of ischemic disease, and occur secondary to infarction of the tip of a papillary muscle. This outcome was seen in 22 patients (mean age, 66.8±10.2 years; median, 66.5 years; range, 53.7-82.7 years) and necessitated a modified Gerbode-Hetzer posterior leaflet plication technique with concomitant coronary revascularization (18). This group was excluded from this study as a different MV repair technique was used.
Table 1 summarizes the basic characteristics of 75 patients in whom the posterior annulus shortening technique was employed.
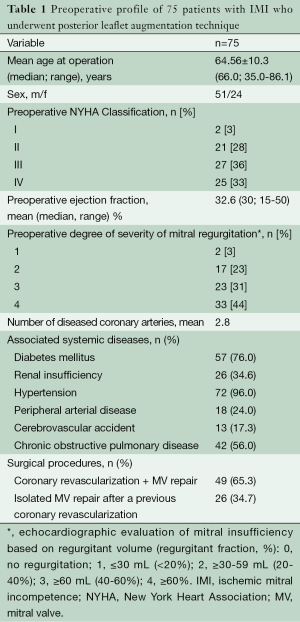
Full table
Imaging protocol and analysis
Echocardiography
Full-volume MV data sets were acquired using real-time two-dimensional echocardiography. Transesophageal echocardiography (TEE) was performed prior to cardiopulmonary bypass and following valve repair. Echocardiographic grading of MV insufficiency was based on regurgitant fraction (RF) and assessed as: 0 (none) = absence of RF; 1 (mild) = RF <20%; 2 (moderate) = RF 20-40%; 3 (moderate to severe) = RF 40-60%; 4 (severe) = RF >60%.
Postoperative analysis was performed at midsystole (frame midway between the first frame demonstrating MV closure and the first frame demonstrating aortic valve closure). The anterior and posterior commissures were defined as annular points at the junction between the anterior and posterior leaflets (middle of commissural region). To trace the coaptation, meticulous care was taken to clearly identify the tip of both the anterior and posterior leaflets immediately before coaptation, so that the highest (most atrial) and lowest (most ventricular) margins of the coaptation zone could be defined. These atrial and ventricular edges of the coaptation zone (actual area of overlap) were then marked interactively. Coaptation length was defined as the extent of overlap between the two leaflets. Any leaflet tissue beyond the area of apposition of two leaflets was not included as coaptation. Mitral annular area was the area enclosed by the two-dimensional projection of a given annular data set onto its corresponding plane. Coaptation area was the area of overlap of the anterior and posterior leaflets across the extent of the entire MV. Posterior leaflet mobility was the difference in the angle subtended by the proximal (annular) portion of the leaflet surface to the line joining the posterior and anterior annulus during systole and diastole. Coaptation distance was the two-dimensional projected distance of the coaptation point from the anterior annulus as a percentage of total anteroposterior annular diameters.
Computed tomography (CT)
Cardiac CT scanning was performed on a 2×128-slice MSCT scanner (Siemens Definition Flash, Erlangen, Germany). A total of 100-120 mL of nonionic contrast medium (Imeron 400, Bracco, Altana Pharma, Konstanz, Germany) was administered via antecubital/jugular vein at 4 mL/s. Automated peak enhancement detection in left atrium was used to time the contrast bolus at threshold level of 160 Hounsfield units. The scan was performed during an inspiratory breath hold of eight seconds. Electrocardiogram was recorded simultaneously to allow retrospective gating and data reconstruction at desired phases of the cardiac cycle. All images were transferred to a processing workstation (Syngovia, Siemens AG) for analysis.
To study the LV anatomy and MV apparatus, the dataset was reconstructed with a slice thickness of 0.75 mm and reconstruction increment of 0.4 mm, starting in early systole (0% of cardiac cycle) to end-diastole (90% of cardiac cycle). All parameters obtained were indexed to body surface area (BSA). MV geometry was studied in the reconstructed mid-systolic phase, when the valve is closed. The annulus area is estimated planimetrically at the level of the MV annulus. Anteroposterior and intercommissural diameters were measured at the level of MV segments A2/P2. The corresponding 3-chamber view as anteroposterior plane through the MV segments A2/P2 was used for measurements of coaptation distance (distance between leaflet coaptation point and mitral annulus plane), tenting area (area between both mitral leaflets under the mitral annulus plane) and MV closure angle (angle between both leaflets at the coaptation point). These measurements possess a detailed analysis of MV anatomy and coherence of leaflets, annulus, subvalvular apparatus including LV and left atrium.
Surgical technique
To approach the MV, we prefer a median sternotomy when additional coronary revascularization is needed and the operation is in favor of a right anterolateral thoracotomy (5th intercostal space) in isolated IMI. After cardiac anesthesia, a Doppler TEE probe is placed and the extent/location of regurgitant jet and severity of MI is noted with assessment of the functional anatomy of the leaflets and subvalvular apparatus. The decision to treat mild regurgitation of ischemic incompetence has been controversial. If, during the intraoperative TEE, a preoperative diagnosis of mild MI disappears after volume loading, the MV would be left alone. However, if the MI were to persist, a repair would be performed. With moderate to severe insufficiency, the decision to perform the MV repair was unquestioned and straightforward. Myocardial protection was mostly provided through blood cardioplegia under normothermia. Through a left atriotomy along the interatrial groove, the annulus, leaflets, chordae tendineae and papillary muscles were exposed and meticulously inspected to determine the precise nature of the lesion. Leaflet coaptation was assessed with a forceful transvalvular saline injection. The anterior and posterior leaflet coaptation, with regard to the presence of sufficient tissues along the coaptation plane, was assessed using a nerve hook. The valve orifice area was measured with a Hegar dilator, and more recently, with a Ziemer-Hetzer valve sizer (Fehling Instruments GmbH, Karlstein, Germany).
In IMI, mitral insufficiency may be caused by dilatation of the annular-ventricular apparatus or dysfunction of the LV wall alongside its papillary muscles or in combination, resulting in failure of leaflet coaptation. As the MV structure appears ostensibly normal in IMI, the lesion is ideal for valve repair.
The posterior annulus shortening technique [modified Paneth-Hetzer posterior annulus shortening (18)] was applied in 75 patients who had an absence of leaflet coaptation, and is performed by running a pericardium-pledgeted 3-0 polypropylene suture through the fibrous body of the trigone and then tied (Figure 1A), after which it is run continuously along the annulus from one trigone towards the midsection of the posterior annulus. The same is done on the opposite trigone. These sutures are then tied over the aforementioned valve sizer to prevent over-narrowing of the valve orifice. When competence is assured, both sutures using the same needles are passed onto an untreated autologous pericardial strip which is then attached to the posterior annulus from the midsegment towards the trigone (Figure 1B) by continuous sutures in a through-and-through fashion. Leaflet coaptation is tested by a forceful transvalvular saline injection to check for residual regurgitation. Shortening the posterior annulus produces a wide and even coaptation, in such a way that when the anterior mitral leaflet closes, the border between the smooth and rough surface of the anterior leaflet forms the closure line with the posterior leaflet without folding (Figure 1C). The posterior annulus is shortened to a degree which reduces the mitral orifice to between 23 and 25 mm in diameter, resulting in a MV orifice of between 3.5 and 4.5 cm2. This residual size is sufficient and ensures a sufficiently broad leaflet coaptation area.
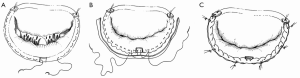
This technique was successful in IMI through augmentation of the posterior annulus by the pericardial strip tissue. The area which the posterior leaflet offers to the anterior leaflet for coaptation during valve closure is enhanced by the pericardial tissue, making valve closure possible even in cases of advanced leaflet restriction (Figure 2A,B).

Evaluation of the adequacy of repair
It is necessary to assess the valve function after MV repair before atrial closure and separation from cardiopulmonary bypass through transvalvular saline injection. Any remaining areas contributing to significant incompetence are attended to before atrial closure. Once de-airing has been completed and extracorporeal circulation is discontinued, the repair result is evaluated with intraoperative TEE to demonstrate adequate mitral orifice, lack of residual incompetence, absence of myocardial ischemia due to coronary kinking and absence of systolic anterior motion (SAM). Precautions are taken to avoid the occurrence of SAM phenomenon, which is seen as “folding” of the anterior leaflet when the valve is tested with transvalvular saline instillation. The folding appears when the valve opening is made too narrow by overshortening of the posterior annulus (19). Immediate correction must be made if the repair is shown to be unsatisfactory. No patient should be discharged from the operating room with anything more than minimal MI.
Follow-up
Follow-up data were provided by the Department of Clinical Studies, Deutsches Herzzentrum Berlin, and written correspondence from the referring physicians and/or telephone interviews with patients or families. No patient was lost to follow-up. Patients underwent routine echocardiography during follow-up visits at the outpatient department and CT scan was performed annually.
Statistical analysis
All data were analyzed with the SPSS 16.0 (SPSS Inc., Chicago Il, USA) software program. Data are expressed as absolute and percentage frequency values and continuous data as mean ± standard deviation, as appropriate. Variables such as NYHA functional class, LV function and severity of mitral insufficiency were analyzed with the Pearson χ2 tests, the two-tailed displayed proportions and odds ratios. Differences among groups were analyzed using the Mann-Whitney U test, log rank (Mantel-Cox) and generalized Wilcoxon. A P value of ≤0.05 was considered significant. Freedom from reoperation and survival rates were analyzed according to Kaplan-Meier estimates with 95% CI.
Results
Mean duration of follow-up was 7.62±0.66 (median, 8.53; range, 3.6-20.9) years.
Change in functional class
Improvement in heart failure symptoms occurred significantly within 6 months to 1 year, and remained stable at 5 years follow-up, and onwards. It is important to note that with the previously described concept of leaflet augmentation for IMI, patients with preoperative NYHA Functional Class III (66.7%) and IV (62.2%) improved to class I on latest follow-up (Figure 3A).
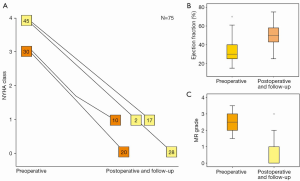
Change in LV ejection fraction
Postoperatively, these patients showed significant improvement in LV ejection fraction at 1 year which was maintained during the course of follow-up (Figure 3B). Using log-rank analysis, there was a statistically significant difference (P<0.001) when the preoperative NYHA and LV ejection fraction were compared to the corresponding postoperative and follow-up parameters.
Change in degree of severity of mitral incompetence
Severity of MI decreased significantly from grade 3.3±0.7 to absent or mild regurgitation of grade 0.42±0.3 (P<0.001) (Figure 3C). Significant early (within 30 days to 6 months) postoperative improvement in the severity of MI was found in 96% of patients. There was no worsening of MI, except in three patients; one of whom had suture dehiscence who then underwent repeat repair, and two underwent MV replacement. The increase in severity of their MI however, did not occur until the late postoperative period. Absent to trivial MI was found in 93% of patients at the latest follow-up.
Imaging analysis of outcome of MV repair
Echocardiography
Leaflet coaptation was restored from zero preoperatively to 6.9±09 cm2 postoperatively. Opening area decreased from a mean of 4.9±0.76 to 3.4±0.78 cm2 postoperatively. The disparity between anterior and posterior mitral leaflets disappeared after repair (Figure 4A,B), leaflet coaptation was restored and insufficiency was abolished (Figure 4C-H).
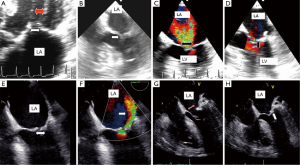
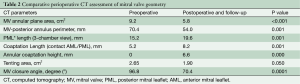
Computed tomography (CT) assessment (Table 2)
Full table
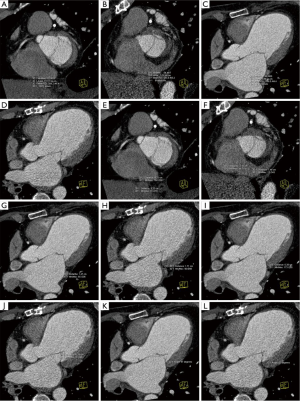
Annular area was reduced from 9.2 to 5.8 cm2 (Figure 5A,B). From a complete absence of coaptation surface, it was increased to 6.6 mm after repair (Figure 5C,D). CT showed posterior annulus size reduction from 70.4 to 54 mm (Figure 5E,F) and an increase in posterior leaflet length from 15.9 to 19.6 mm (Figure 5G,H). A remarkable CT finding was the increase in coaptation length from 5.2 to 8.2 mm (Figure 5I,J). MV closure angle decreased from preoperative 96.8° to 70.4° postoperatively (Figure 5K,L).
Freedom from reoperation
Overall freedom from reoperation was 97.3%±1.9%, 95.3%±2.7% and 86.4%±5.6% at 30 days, 1 and 5 years onwards, respectively (Figure 6A). Freedom from reoperation was 96.2%±3.8% in patients who underwent posterior leaflet augmentation technique alone compared to 92.3%±4.2% in patients with concomitant coronary revascularization (Figure 6B).
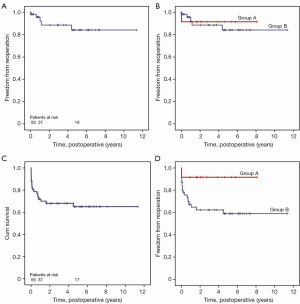
Reoperation consisted of MV replacement in two patients at 1 and 5 years after the initial repair, and one patient was reoperated due to annular suture dehiscence 3 years later. Two reoperations unrelated to the MV repair consisted of ventricular assist device implantation and another two patients underwent orthotopic heart transplantation a year later. These were the patients who preoperatively had ejection fraction of 15%, NYHA functional class IV and MI of grade four, with associated triple-vessel disease.
Survival
Overall survival rate was 84.9%±4.2%, 71.9%±5.5% and 65.1%±6.3% at 30 days, 1 and 5 years onwards, respectively (Figure 6C). Survival rate was 60.3%±1.8% in patients who underwent isolated MV repair and 93.5%, in patients who underwent MV repair with concomitant coronary revascularization (Figure 6D).
Discussion
Surgical management of IMI has primarily consisted of revascularization with or without addition of MV repair with a variety of techniques (20-25) including suture, band/ring annuloplasty or MV replacement. However, these still leave 30% of patients with residual or recurrent mitral insufficiency (4-8).
Given the fact that IMI is in essence a “ventricular and not a valvular disease”, the posterior leaflet augmentation technique by posterior annulus shortening targets the consequence—mitral insufficiency from absence of leaflet coaptation around the posterior parts of both the anterior and/or posterior leaflets, seen as leaflet tethering and lack of leaflet coaptation. Use of autologous pericardial strip to stabilize the shortened posterior annulus not only reinforces the repair, but helps in maintaining the MV geometry. This strategy offers an incremental benefit over standard annuloplasty or implantation of prosthetic devices, in that shortening the posterior annulus reduces the LV sphericity, thereby improving the ventricular shape and size along with reductions in subvalvular apparatus tethering and interpapillary muscle distance. In effect, this geometrical restoration helps improve LV function.
In this series, we have shown a hemodynamic advantage in performing posterior annulus shortening technique. There was very low incidence of residual or recurrent MI. We believe that these results may be due to the restoration of annular shape and preservation of the subvalvar apparatus. By increasing the coaptation area, the prolapse of the anterior leaflet during systole, a common mechanism in recurrent IMI, is prevented.
This repair technique reduces the mitral orifice to a residual size sufficient to ensure a sufficiently broad leaflet coaptation area. The primary goal is to achieve complete and rapid closure of the mitral orifice by an adequately mobile anterior leaflet and a sufficiently large coaptation area, by bringing the posterior leaflet closer to the anterior leaflet. This technique is effective through a mechanism by the augmentation of the posterior annulus caused by the pericardial strip tissue, which we have previously explained. This means that during valve closure, the area that the posterior leaflet offers to the anterior leaflet for coaptation is enlarged and heightened by the strip tissue, making valve closure possible even in advanced leaflet restriction.
Conclusions
In patients with IMI, posterior annulus shortening with pericardial strip augmentation by moving the posterior leaflet closer to the anterior leaflet is a simple, reproducible, reliable and effective repair technique to restore valve competence. It addresses the pathophysiology of lack of leaflet coaptation and provides satisfactory long-term functional outcome.
Acknowledgements
We thank Anne Gale, medical editor, for assistance with this article. We also appreciate the assistance of Christine Detschades, Julia Stein, Carla Weber and Helge Haselbach.
Disclosure: The authors declare no conflict of interest.
References
- Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. [PubMed]
- Otsuji Y, Handschumacher MD, Liel-Cohen N, et al. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol 2001;37:641-8. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:e523-661. [PubMed]
- McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916-24. [PubMed]
- Hung J, Papakostas L, Tahta SA, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation 2004;110:II85-90. [PubMed]
- Matsunaga A, Tahta SA, Duran CM. Failure of reduction annuloplasty for functional ischemic mitral regurgitation. J Heart Valve Dis 2004;13:390-7; discussion 397-8. [PubMed]
- Gelsomino S, Lorusso R, De Cicco G, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J 2008;29:231-40. [PubMed]
- Tahta SA, Oury JH, Maxwell JM, et al. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis 2002;11:11-8; discussion 18-9. [PubMed]
- Kuwahara E, Otsuji Y, Iguro Y, et al. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: importance of augmented posterior leaflet tethering. Circulation 2006;114:I529-34. [PubMed]
- Salgo IS, Gorman JH 3rd, Gorman RC, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation 2002;106:711-7. [PubMed]
- Borger MA, Murphy PM, Alam A, et al. Initial results of the chordal-cutting operation for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2007;133:1483-92. [PubMed]
- Rama A, Praschker L, Barreda E, et al. Papillary muscle approximation for functional ischemic mitral regurgitation. Ann Thorac Surg 2007;84:2130-1. [PubMed]
- Robb JD, Minakawa M, Koomalsingh KJ, et al. Posterior leaflet augmentation improves leaflet tethering in repair of ischemic mitral regurgitation. Eur J Cardiothorac Surg 2011;40:1501-7; discussion 1507. [PubMed]
- Jassar AS, Minakawa M, Shuto T, et al. Posterior leaflet augmentation in ischemic mitral regurgitation increases leaflet coaptation and mobility. Ann Thorac Surg 2012;94:1438-45. [PubMed]
- de Varennes B, Chaturvedi R, Sidhu S, et al. Initial results of posterior leaflet extension for severe type IIIb ischemic mitral regurgitation. Circulation 2009;119:2837-43. [PubMed]
- Langer F, Rodriguez F, Cheng A, et al. Posterior mitral leaflet extension: an adjunctive repair option for ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2006;131:868-77. [PubMed]
- Kincaid EH, Riley RD, Hines MH, et al. Anterior leaflet augmentation for ischemic mitral regurgitation. Ann Thorac Surg 2004;78:564-8; discussion 568. [PubMed]
- Hetzer R, Delmo Walter EM. No ring at all in mitral valve repair: indications, techniques and long-term outcome. Eur J Cardiothorac Surg 2014;45:341-51. [PubMed]
- Hetzer R, Delmo Walter EM. Repair of Congenital Mitral Valve Insufficiency. Oper Tech Thorac Cardiovasc Surg 2010;15:260-72.
- Rothenburger M, Rukosujew A, Hammel D, et al. Mitral valve surgery in patients with poor left ventricular function. Thorac Cardiovasc Surg 2002;50:351-4. [PubMed]
- Szalay ZA, Civelek A, Hohe S, et al. Mitral annuloplasty in patients with ischemic versus dilated cardiomyopathy. Eur J Cardiothorac Surg 2003;23:567-72. [PubMed]
- Chen FY, Adams DH, Aranki SF, et al. Mitral valve repair in cardiomyopathy. Circulation 1998;98:II124-7. [PubMed]
- Lachmann J, Shirani J, Plestis KA, et al. Mitral ring annuloplasty: an incomplete correction of functional mitral regurgitation associated with left ventricular remodeling. Curr Cardiol Rep 2001;3:241-6. [PubMed]
- Bolling SF. Mitral reconstruction in cardiomyopathy. J Heart Valve Dis 2002;11:S26-31. [PubMed]
- Silberman S, Klutstein MW, Sabag T, et al. Repair of ischemic mitral regurgitation: comparison between flexible and rigid annuloplasty rings. Ann Thorac Surg 2009;87:1721-6; discussion 1726-7.





