Lifting posterior mitral annuloplasty for enhancing leaflet coaptation in mitral valve repair: midterm outcomes
Introduction
Mitral annuloplasty has been an important component of most mitral valve (MV) repair techniques for mitral valve regurgitation (MR) (1,2). Mitral valve annular geometry has been studied to maintain the annular motion and improve repair outcomes after MV annuloplasty (3-5). However, most traditional rigid, semi-rigid, or flexible rings have been affixed to the valve annulus on the flat plane, with limited annular motion during the cardiac cycle (4). Although some flexible rings have been manufactured to allow native annular motion, both septal-lateral and transverse dimensions of the valve become nearly motionless because the entire annulus is affixed to the ring. For two-leaflet coaptation, reduction of the transverse valve dimension by annuloplasty may be unnecessary and could disturb the coaptation. However, reduction of the septal-lateral dimension may be an important factor. In animal models, reduction of the septal-lateral annular dimension and an increased mitral hinge angle have been the main factors for facilitating mitral leaflet coaptation (6). The annuloplasty ring shape has also been emphasized in producing annular non-planarity and leaflet curvature in order to reduce valvular stress and increase repair durability (4).
A novel mitral annuloplasty strip was designed to lift the middle portion of the posterior annulus on the basis of both commissural ends, sparing the anterior leaflet and the commissures. This study evaluated the mid-term results of the innovative lifting mitral annuloplasty (LPMA), which was performed to complete MV repair in patients with MR caused by various etiologies.
Methods
This study is a retrospective nonrandomized review of the prospective follow-up of all MR patients who underwent LPMA for MV repair. This study was approved by the Institutional Review Board at Konkuk University.
Patients
Between October 2007 and December 2012, 341 consecutive patients (mean age, 50.7±14.8 years; 168 men/173 women) who had significant MR underwent LPMA with the novel annuloplasty strip (Figure 1) after providing written informed consent at Konkuk University Medical Center (Table 1). The causes of MR were degeneration in 235 patients (68.9%), rheumatism in 29 patients (8.5%), endocarditis in 22 patients (6.5%), cardiomyopathy in 21 patients (6.2%), ischemia in 21 patients (6.2%), and secondary to congenital heart disease in 13 patients (3.8%). Three hundred and fourteen patients (92.1%) exhibited substantial moderate to severe MR, and 27 (7.9%) had moderate MR. Of the patients with moderate MR, 19 had mixed stenosis and regurgitation of rheumatic origin, five had ischemic MR, and three had MR associated with congenital heart diseases.
Full table
During the study period, 450 patients required primary mitral valve surgery. Of them, only five patients (1.1%) underwent valve replacement. The remaining 445 patients underwent LPMA associated with MV repair. Of them, 104 patients with concomitant aortic valve surgery or commissurotomy for pure mitral stenosis without MR were excluded, since the aim of the study was to assess the effect of LPMA in patients with MR.
Operative technique
Most patients (n=277, 81.2%) underwent MV surgery through a right thoracotomy, and the remaining patients (n=64, 18.8%) through a median sternotomy. Cardiopulmonary bypass was established with extrathoracic arterial (femoral or axillary artery) and venous cannulation (femoral vein and right internal jugular vein) for cases of right thoracotomy, or with standard aortic and direct vena cava cannulation for cases of median sternotomy under moderate hypothermia. After achieving cardiac arrest with an infusion of cold blood cardioplegic solution, the MV was exposed through a left atriotomy in the Sondergaard groove.
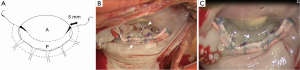
The LPMA technique was described in detail previously (7). Prior to LPMA, patch valvuloplasty for posterior leaflet prolapse (8), placement of new chords for anterior leaflet prolapse, leaflet extension for tethered posterior leaflet, and/or the Cox-Maze operation for atrial fibrillation were performed. The size of the annuloplasty strip was determined by measuring the intercommissural distance. The strip was placed in the supra-annular portion along the posterior annulus; neither the anterior annulus nor the commissures were involved. Six braided 2-0 Dacron sutures were used to fix the strip in position (Figure 2A,B). For posterior leaflet extension, the posterior leaflet was detached from the annulus; a 3.0-mm leaflet margin remained intact at both commissures. The leaflet defect was closed with an elliptical bovine pericardial patch 13 to 15-mm in width using running 4-0 Prolene (Ethicon, Somerville, NJ, USA) sutures.
Posterior annuloplasty strip
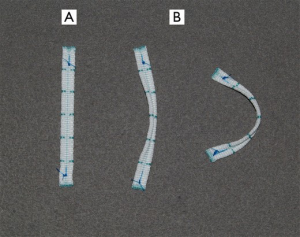
The LPMA strip was a flat 5.0-mm-wide strip made of Dacron with a thin central gully between two thick margins. The strip is flexible in the flat plane, and relatively rigid in the vertical plane due to its thick margins (Figure 1). Commonly used strip lengths were 53, 55, 58, and 61 mm for intercommissural dimensions of 30, 32, 34, and 36 mm, respectively (Table 2).
Full table
After LPMA, the posterior annular length was reduced to 1.5 times the anterior annular length by the appropriately-sized strip (9). The strip and posterior annulus became a curvilinear complex in which the middle portion was lifted above the horizontal plane of both commissures and was pushed forward in view of the vertical plane (Figure 1, Figure 2B,C).
Associated procedures for MV repair and concomitant cardiac surgeries
Twenty-nine patients (8.5%) with rheumatic mixed stenosis and regurgitation underwent commissurotomy, decalcification, stripping of the thickened leaflets, and penetration of fused chords. Anterior leaflet prolapse (chord elongation or rupture) was repaired by the placement of artificial chords (4-0 or 5-0 polytetrafluoroethylene sutures) in 33 patients (9.7%). Posterior leaflet prolapse (chord elongation or rupture) was repaired by patch valvuloplasty (8) in 80 patients (23.5%) and quadriangular resection in 4 patients (1.2%).
Posterior leaflet extension was performed in 19 rheumatic patients with mixed stenosis and regurgitation and four patients with ischemic MR. Two patients also underwent anterior leaflet extension for short anterior leaflets. The MV repair was associated with other cardiac procedures in 129 cases (37.8%): tricuspid annuloplasty in 50, Cox maze procedure in 104, coronary artery bypass graft in 24, and congenital cardiac defect repair in 9 (Table 2).
Echocardiographic measurements
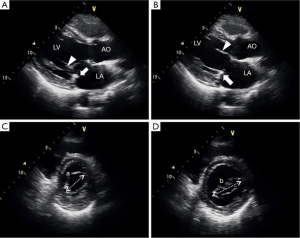
Transthoracic echocardiography was performed at admission, discharge, 6-months postoperatively, and annually. Transesophageal echocardiography (10) was performed at admission and during the operation. MR grade was determined according to the following scale: 0, no or trivial MR; 1+, mild; 2+, moderate MR; 3+, moderately severe MR; and 4+, severe MR. The MV orifice area was assessed by the pressure half-time method (11). The intercommissural dimensions were measured in early and late diastole to investigate changes in the dimensions. When the valve was fully opened (early diastole), the septal-lateral and intercommissural dimensions were measured. In the parasternal long-axis view, the coaptation height (the longest coaptation length of the anterior leaflet) and depth (the shortest distance between the coaptation and annular plane) were measured in early systole.
Statistical analysis
All statistical analyses were performed in SPSS 18.0 (IBM, Armonk, NY, USA). Continuous variables were expressed as the mean ± standard deviation and compared using the Student’s t-test and paired t-test, and categorical variables were expressed as proportions (%) and compared using the χ2 test. Survival and freedom from progression of MR or reoperation probability were estimated using the standard non-parametric Kaplan-Meier method.
Results
Morbidity and mortality
Three (0.9%) hospital deaths (≤30 days) occurred in the study population. The causes of the three deaths were non-cardiac: gastrointestinal bleeding, intracranial bleeding and pneumonia, and sepsis. During a mean follow-up period of 38.2±17.1 months, nine late deaths (2.6%) occurred, all between postoperative day 37 and 2.9 years. The cause of death could be assessed in seven of the nine patients. Two patients died of cardiac-related causes: left ventricular (LV) failure in one and right heart failure in another. The other five patients died of non-cardiac causes: esophageal perforation in one, pneumonia in two, cerebrovascular accident in one, and traffic accident in one. Overall survival at 5-years was 96.0%±1.1%.
Follow-up data
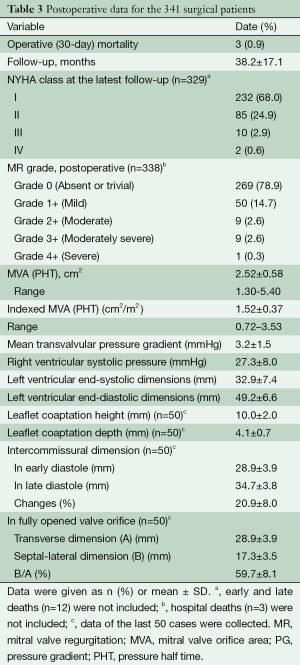
The mean echocardiographic follow-up time was 23.2±18.2 months. At the last echocardiographic examination of 338 early survivors, MR was absent or trivial in 269 patients (78.9%), mild in 50 patients (14.7%), moderate in nine patients (2.6%), moderately severe in nine patients (2.6%), and severe in one patient (0.3%). The one patient with severe recurrent MR underwent reoperation for valve replacement on postoperative day 9. Mean freedom from recurrence of MR ≥3+ at 5 years was 95.1%±1.6%.
Twenty-three patients (19 with rheumatic MR and four with ischemic MR) who underwent posterior leaflet extension had no or trivial MR. Of the 29 patients who underwent concomitant commissurotomy, five had moderate stenosis.
In an echocardiographic follow-up of 285 patients (83.6%), the MV area and MV area index were 2.5±0.6 cm2 and 1.5±0.4 cm2/m2, respectively, whereas the mean transvalvular pressure gradient (PG) was 3.2±1.5 mmHg. All patients exhibited coaptation below the posterior annulus and the strip (Figure 2C, Figure 3A,B).
Geometry of the MV after LPMA was analyzed by two-dimensional echocardiography for the last 50 patients. The mean leaflet coaptation depth and height in early systole were 4.1±0.7 and 10.0±2.0 mm, respectively (Table 3). Between early and late diastole, the mean change in intercommissural dimensions was 20.9%±8.0% (34.7±3.8 mm in late diastole vs. 28.9±3.9 mm in early diastole; P<0.0001; Figure 3C,D). When the valve fully opened (at the largest septal-lateral dimension), the mean ratio of the septal-lateral and inter-commissural dimensions was 59.7%±8.1% (Table 3). The mean LV end-systolic and diastolic dimensions decreased from 37.5±8.3 to 32.9±7.4 mm and from 57.3±8.1 to 49.2±6.6 mm (P<0.001), respectively.
Full table
Reoperation
Reoperation was performed in nine patients (2.6%; overall 5-year freedom rate of reoperation, 97.0%±1.0%). Six patients (1.8%) underwent reoperation due to recurrent MR: five patients underwent valve repair with LPMA and one patient underwent MV replacement with a 27-mm pericardial valve. Mean freedom from valve-related reoperation at 5-years was 98.1%±0.8%. The remaining three patients underwent non-valve-related reoperations: two patients underwent cardiac transplantation due to aggravated cardiomyopathy and one patient underwent tricuspid valve replacement and right ventricular reduction. This last patient died from right heart failure on postoperative day 1.
Discussion
The present study reports midterm results for LPMA with the new posterior annuloplasty strip in a series of patients with MR. During the study period, primary MV replacement was performed in only five patients. The main finding of this study is that mean freedom from recurrence of MR grade 3+ or 4+ was 95.1%±1.6% at 5-years, and mean freedom from valve-related reoperation was 98.1%±0.8% at 5-years, despite the fact that most patients (98.9%) who required primary MV surgery underwent MV repair with LPMA. The freedom from progression to moderate or severe MR and freedom from valve-related reoperation after MV repair in our series were comparable to previous results (12-14). The echocardiographic follow-up of LPMA combined with other valve repair procedures, including new chord placement, patch valvuloplasty (8), and leaflet extension, showed a favorable result, with recurrent MR ≥3+ in only 10 patients (2.9%). Five-year freedom of 99% from severe MR was lower than the linear recurrence rate of 1.5% to 2% per year in previous annuloplasty results (13-15). The operative mortality of 0.9% is comparable to previously reported results (13,15-17), despite the same LPMA procedure being used for most patients with MR, including mixed stenosis and regurgitation of rheumatic origin.
Post-repair residual MR is significantly associated with the absence of a prosthetic annuloplasty (13,15). To obtain adequate leaflet coaptation and prevent recurrent MR, several types of annuloplasty prostheses have been designed. However, a consensus regarding the optimal design for each valvular disease has not been reached (6). The LPMA strip is a flexible Dacron strip, and its structure is quite different from that of the traditional rings. The strip is flexible when flat, but rigid in the vertical direction due to two thick margins. As the strip is placed along the posterior annulus only, the strip is several millimeters shorter than the traditional flexible c-type rings. After its supraannular placement, the strip becomes curvilinear due to the round nature of the posterior annulus, and lifts and pushes the middle portion of the posterior annulus forward. The strip also has a straightening force due to its two thickened margins. The functional concept of the strip, which reduces the septal-lateral dimension and lifts the middle portion of the posterior leaflet, is different from rigid rings that were used to treat degenerative MR. After LPMA, however, the preserved anterior annulus and commissures and the flexible strip allow change in the intercommissural distance when the valve opens and closes during the cardiac cycle. During systole, the anterior leaflet and commissures preserved by LPMA may maintain their native annular hinge motion, which is an important factor for facilitating leaflet coaptation (6). Additionally, the posterior leaflet lifted by LPMA may increase the leaflet curvature, which is also an important mechanism for stress reduction (5). As the middle portion of the posterior annulus is lifted and pushed forward, the septal-lateral dimension of the valve is reduced, which enhances leaflet coaptation (6,18). LPMA after mitral commissurotomy did not significantly reduce the valve orifice area. Furthermore, the straightening force produced by two thick margins of the strip may enhance commissural coaptation after commissurotomy, when there are no chords. Among our cases of mitral commissurotomy and LPMA, none exhibited regurgitation flow in commissural corners. In echocardiography, the leaflet coaptation was always below the annuloplasty strip. The supra-annular position and flat shape of the strip seem to be reasons for the lack of hemolytic complications in cases of remnant MR.
The low freedom from valve-related reoperation (98.1%±0.8% at 5-years) means that the LPMA procedure results in favorable leaflet coaptation and rare recurrence of MR. In other words, the anterior annulus and commissures that were not involved in the annuloplasty are not insidious structures that progressively dilate and induce recurrent MR. Therefore, we suggest preserving the anterior annulus and commissures when performing annuloplasty to retain their native motion during the cardiac cycle.
The postoperative valve area and mean MV gradient were similar to the previously reported data (19), but the real valve area may have been underestimated by echocardiography, as the pressure half time (PHT) can be measured relatively high in the central valve portion with a reduction of the septal-lateral dimension. In early diastole, however, the septal-lateral dimension was significantly increased by full opening of the anterior leaflet while the intercommissural dimension was reduced. Although the LPMA had several causative factors for systolic anterior motion (SAM) (20), such as reduced septal-lateral dimension, increased coaptation height, and posterior leaflet extension, SAM never occurred because the LPMA technique did not disturb or affect the motion of the anterior leaflet and annulus. Further prolapse secondary to repair failure was not seen after LPMA.
Ring fixation to the commissures is not required to enhance the coaptation of two leaflets and prevent annular re-dilatation. For myxomatous mitral valve diseases in which systolic saddle—shape deepening (accentuation) is not recovered after annuloplasty (21), LPMA may compensate for the lost systolic saddle-shape via posterior annular lifting.
This retrospective analysis has several limitations. First, valve geometric data were collected from a limited number of the patients. Second, this study did not measure the hinge angle that defines the annular saddle motion and leaflet curvature. To testify to the advantages of LPMA, the annular motion should be confirmed by 3D echocardiographic measurements. Finally, the clinical outcome of LPMA may be affected by additional repair procedures, such as patch valvuloplasty and the placement of new chords. The long-term durability of LPMA and survival should be followed up in future studies.
Conclusions
In mitral valve repair of variable types of mitral regurgitation, lifting posterior mitral annuloplasty for enhancing leaflet coaptation had a low rate of recurrent regurgitation or valve-related reoperation with rare relevant complications. Thus, mitral annuloplasty using an innovative annuloplasty strip can be quite useful for completing mitral valve repair in most patients who have mitral regurgitation.
Acknowledgements
Funding: This work was supported by Fund of Biomedical Research Institute of Chonbuk National University Hospital and Fund of Konkuk University Medical Center in 2014.
Disclosure: The authors declare no conflict of interest.
References
- Durán CM, Pomar JL, Cucchiara G. A flexible ring for atrioventricular heart valve re-construction. J Cardiovasc Surg (Torino) 1978;19:417-20. [PubMed]
- Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation 2001;104:I8-11. [PubMed]
- Ryan LP, Jackson BM, Hamamoto H, et al. The influence of annuloplasty ring geome-try on mitral leaflet curvature. Ann Thorac Surg 2008;86:749-60; discussion 749-60. [PubMed]
- Mahmood F, Gorman JH 3rd, Subramaniam B, et al. Changes in mitral valve annular geometry after repair: saddle-shaped versus flat annuloplasty rings. Ann Thorac Surg 2010;90:1212-20. [PubMed]
- Hu X, Zhao Q. Systematic evaluation of the flexible and rigid annuloplasty ring after mitral valve repair for mitral regurgitation. Eur J Cardiothorac Surg 2011;40:480-7. [PubMed]
- Itoh A, Ennis DB, Bothe W, et al. Mitral annular hinge motion contribution to changes in mitral septal-lateral dimension and annular area. J Thorac Cardiovasc Surg 2009;138:1090-9. [PubMed]
- Choi JB, Kim KH, Kim MH, et al. Improvement of mitral valve coaptation with su-praannular plication of the posterior annulus--a newly designed strip for posterior annular plication--. Ann Thorac Cardiovasc Surg 2012;18:95-100. [PubMed]
- Chung JW, Shin JK, Song MG, et al. Patch valvuloplasty in patients with posterior chordae rupture. Int J Cardiol 2012;154:206-7. [PubMed]
- Duplessis LA, Marchand P. The anatomy of the mitral valve and its associated struc-tures. Thorax 1964;19:221-7. [PubMed]
- Saiki Y, Kasegawa H, Kawase M, et al. Intraoperative TEE during mitral valve repair: does it predict early and late postoperative mitral valve dysfunction? Ann Thorac Surg 1998;66:1277-81. [PubMed]
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve ste-nosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23. [PubMed]
- Gillinov AM, Tantiwongkosri K, Blackstone EH, et al. Is prosthetic anuloplasty nec-essary for durable mitral valve repair? Ann Thorac Surg 2009;88:76-82. [PubMed]
- Nardi P, Pellegrino A, Scafuri A, et al. Survival and durability of mitral valve repair surgery for degenerative mitral valve disease. J Card Surg 2011;26:360-6. [PubMed]
- Meyer MA, von Segesser LK, Hurni M, et al. Long-term outcome after mitral valve repair: a risk factor analysis. Eur J Cardiothorac Surg 2007;32:301-7. [PubMed]
- Flameng W, Meuris B, Herijgers P, et al. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg 2008;135:274-82. [PubMed]
- DiBardino DJ, ElBardissi AW, McClure RS, et al. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010;139:76-83; discussion 83-4. [PubMed]
- Gillinov AM, Mihaljevic T, Blackstone EH, et al. Should patients with severe degenerative mitral regurgitation delay surgery until symptoms develop? Ann Thorac Surg 2010;90:481-8. [PubMed]
- Tibayan FA, Rodriguez F, Langer F, et al. Annular remodeling in chronic ischemic mi-tral regurgitation: ring selection implications. Ann Thorac Surg 2003;76:1549-54; discussion 1554-5. [PubMed]
- Vohra HA, Whistance RN, Bezuska L, et al. Initial experience of mitral valve repair using the Carpentier-Edwards Physio II annuloplasty ring. Eur J Cardiothorac Surg 2011;39:881-5. [PubMed]
- Cohn LH, McClure RS. Acquired valvular heart disease. In: Kaiser L, Kron IL, Spray TL. eds. Mastery of Cardiothoracic Surgery. 3rd ed. Philadephia: Wolters-Kluer, 2014:414-7.
- Grewal J, Suri R, Mankad S, et al. Mitral annular dynamics in myxomatous valve dis-ease: new insights with real-time 3-dimensional echocardiography. Circulation 2010;121:1423-31. [PubMed]





