Evolution of the concept and practice of mitral valve repair
Closed mitral valve repair
The first successful valve surgery of any kind occurred at Peter Bent Brigham Hospital in 1923. This hospital, which is now known as Brigham and Women’s Hospital, was opened in 1913 with Dr. Harvey W. Cushing as the first surgical chair. Besides his primary interest in neurosurgery, Cushing had also been interested in the relief of mitral stenosis for many years while working at Johns Hopkins Hospital in Baltimore. In 1923, Dr. Elliot C. Cutler, who was one of Cushing’s trainees, performed the first successful mitral valve repair. The patient was a young girl with rheumatic mitral valve stenosis who was comatose from low cardiac output. Cutler pushed a knife through the apex of the left ventricle, then encountered the mitral orifice and performed a blind mitral commissurotomy. The patient was discharged from the hospital a few days later. This case was reported in the Boston Medical and Surgical Journal, which is now known as the New England Journal of Medicine, six weeks later (1). Cutler later went on to develop a device to relieve mitral stenosis. Unfortunately, its principle was to excise a large portion of the mitral valve, which of course resulted in severe mitral regurgitation. All of his subsequent patients died and he discontinued mitral valve repair in 1929. In 1925, Sir Henry S. Souttar performed the first finger fracture of mitral stenosis. After the Second World War, Dr. Dwight E. Harken, the first chief of cardiac surgery at the Peter Bent Brigham Hospital, performed a large series of closed mitral valvuloplasties for patients with mitral valve stenosis. Harken had also become famous for removing shell fragments lodged in soldiers’ hearts and lungs. In 1973, Harken reported a twelve-year follow-up of almost 1,600 patients with closed valvuloplasty (2). He worked very closely with Dr. Laurence B. Ellis, who was a cardiologist at Peter Bent Brigham Hospital. This exemplifies the concept that mitral valve problems are best treated by a team involving cardiac surgeons, cardiologists and cardiac anesthesiologists working together.
Early stages of open mitral valve repair
The first mitral valve repair for mitral insufficiency was performed by Dr. C. Walton Lillehei at the University of Minnesota in 1957 (3,4). Subsequently, the forerunner of the modern techniques for mitral valve repair was reported by Dr. Dwight C. McGoon of the Mayo Clinic in the Journal of Thoracic and Cardiovascular Surgery in 1960 (5). A technique to repair a ruptured cord in the posterior leaflet of the mitral valve was described. The first artificial mitral valve was implanted by Dr. Nina Starr Braunwald at the National Institutes of Health. This valve was a home-made device and was never produced commercially. Mitral valve surgery was subsequently revolutionized by Dr. Albert Starr and his collaborator M. Lowell Edwards at the University of Oregon, who developed the first commercially successful artificial mitral valve in the early 1960s.
One of the early misconceptions about mitral valve surgery was reinforced by a report in the 1970s, suggesting that in some patients the sudden return of competence of the mitral valve has a deleterious effect on left ventricular function and forward flow early postoperatively (6). Unfortunately, this resulted in delayed referral of patients who were very far along in the natural history of the disease because cardiologists were concerned that abrupt closure of the mitral valve would injure the ventricle. Dr. Miller and colleagues at Stanford University then demonstrated the importance of the mitral apparatus in maintaining good ventricular function after mitral valve surgery. This means that the chordae and the papillary muscles should remain intact, which is obviously best achieved by mitral valve repair (7-9). In the 1980s, there was an increased incidence of mitral valve repair, primarily as a result of improved myocardial protection, the recognition that papillary muscle integrity is critical to maintain good ventricular function and long-term data with reconstructive techniques showing excellent outcomes. In contrast, late results with bioprosthetic and prosthetic valves were good but still had a lot of problems. For example, the Hancock valve was the first porcine valve developed, but data showed that these valves degenerated in time (10) and that mitral valves needed to be repaired whenever possible.
Evolution of mitral valve repair
In 1983, Dr. Alain F. Carpentier of the University of Paris published a seminal paper called “The French Correction” in the Journal of Thoracic and Cardiovascular Surgery (11). This paper outlined the basic pathophysiological classification of mitral valve lesions and provided the tools and essentially a game plan for how to successfully and reproducibly repair mitral valve regurgitation, particularly from degenerative myxomatous mitral valve disease. Many surgeons throughout the world were inspired by this paper to perform mitral valve repair operations. The increasing success rate of mitral valve repair subsequently resulted in earlier referral of patients with mitral valve disease for surgical repair. Thus, repair became by far the most frequent mitral valve operation done at the Brigham and Women’s Hospital. The American College of Cardiology also included in its guidelines that mitral valve repair may be performed in asymptomatic patients with normal left ventricular function, if performed by an experienced surgical team and that the likelihood of successful mitral valve repair was greater than 90% (12).
The success of modern mitral valve repair depended on the concept of the interactive service line, a collaborative network of cardiology, cardiac surgery and cardiac anesthesia working together. At Brigham and Women’s Hospital, cardiovascular anesthesia in particular has taken a prominent role in assessing the anatomy, physiology, safety and efficacy of the mitral valve repair procedures that are performed. Using echocardiography in the operating room, cardiac anesthesiologists can locate the circumflex coronary artery, detect potential for systolic anterior motion (SAM), clarify the anatomy of the posterior and anterior mitral valve leaflets, measure the intertrigonal distance, and reveal a number of other anatomic and physiologic factors that are important for mitral valve repair. For example, the course of the circumflex coronary artery near the posterior leaflet has resulted in disastrous complications when surgeons did not understand this anatomical relationship.
Modern techniques for mitral valve repair
One of the key principles for repair of the myxomatous mitral valve is that “less is more”. The basic anatomic problem should be relieved first. Once this is done, one should resist the temptation to do more than one needs.
Posterior leaflet resection
The classic operation designed by Carpentier involved cutting out the ruptured cord segment of the posterior leaflet and creating advancement flaps of the whole posterior leaflet of the mitral valve (Figure 1A). However, many surgeons were worried that cutting the posterior leaflet of the annulus could result in complications. In contrast, the technique developed at Brigham and Women’s Hospital involves excising a small piece of the myxomatous valve and then simply folding it over without doing a major advancement. In comparison with cutting away the entire posterior leaflet, this technique is both faster and much safer, while accomplishing exactly the same principle (Figure 1B).
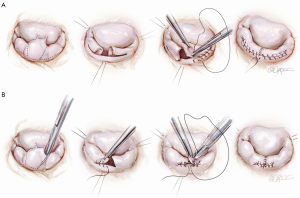
Foldoplasty and commissuroplasty
Another simplified technique for mitral valve repair is called a foldoplasty. This involves repair of an isolated prolapse of the P2 segment of the posterior leaflet by folding over the leaflet to reduce its effective height (Figure 2). Similarly, valve damage in the area of the commissures can simply be repaired by a commissuroplasty, which obliterates this part of the valve with mattress sutures rather than resecting and reconstructing this part of the valve (Figure 3). Mitral valves, in particular degenerative myxomatous mitral valves, have a large orifice and can be safely and efficiently repaired in this way to reduce regurgitation.
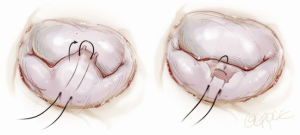
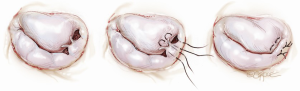
Annuloplasty rings
It has also become clear that annuloplasty rings are virtually always needed in mitral valve repair. The annuloplasty rings should be sized to the height of the anterior leaflet, not from trigone to trigone. Dr. Carlos M. G. Duran pioneered flexible rings for the dynamic annulus (13). Early results from Brigham and Women’s Hospital showed a dramatic difference in the recurrence rates between mitral valve repairs with and without an annuloplasty ring. Specifically, patients with regurgitant myxomatous mitral valves that underwent repair with a ring had a 3.6% recurrence rate, which contrasts with a recurrence rate of 15% for valves that underwent repair without a ring (14). This showed that an annuloplasty rings are needed in myxomatous disease of the mitral valve.
Systolic anterior motion (SAM)
SAM is caused by asymmetry of the anterior leaflet and represents one of the most concerning and complex problems that occur after mitral valve repair. In this condition, the anterior leaflet obstructs the left ventricular outflow tract. The most straightforward technique to limit persistent SAM after complex mitral valve repairs is an edge-to-edge repair. Other techniques to limit SAM are more complicated and involve, for example, the use of additional chords. However, in myxomatous valves, which have very large orifices, an edge-to-edge repair is probably the simplest and most straightforward technique to obliterate SAM and to move the operation along (15). At Brigham and Women’s Hospital, edge-to-edge repair is used if there is a potential for SAM or severe SAM following mitral valve repair, but is not used to correct mitral regurgitation following mitral valve repair that is unrelated to SAM.
Anterior leaflet repair
For repair of the mitral valve anterior leaflet, we have utilized Gore-Tex chordae (W. L. Gore & Associates, Inc., Medical Products Division, Flagstaff, Arizona, USA) or a very limited anterior leaflet resection. Anterior leaflet Gore-Tex chordal repair must be done to make the heights of the anterior and posterior leaflets equivalent to ensure that they coapt precisely after the heart resumes beating.
However, anterior mitral valve repair is not always necessary in bileaflet mitral valve prolapse. In 90% of patients with bileaflet mitral valve prolapse and no specific anatomic lesion of the anterior leaflet, a posterior leaflet repair and annuloplasty are sufficient to have a competent valve without SAM in the vast majority of cases (16). This represents another example of the principle that surgeons should only do what they really have to do, and resist the temptation to do more than necessary. Anterior leaflet prolapse will be resolved by a good posterior leaflet repair and annuloplasty.
Etiology and outcomes
A personal series of 1,503 mitral valve repairs done at Brigham and Women’s Hospital from 1972 to 2008 has shed some light on the relationship between the etiology of mitral valve regurgitation and the outcomes of mitral valve repair (17). This discussion will focus on three different disease states: myxomatous mitral valve disease, functional mitral regurgitation (FMR), and rheumatic mitral valve disease.
Patients in this series had a mean age of 60, and 43% were females. The 30-day mortality in this series was 1.3% without a difference among the four decades of performance. FMR had a higher mortality at 4.7% and the other two had mortality less than 1%. Thus, while myxomatous valves do best and rheumatic valves fare almost the same, there are worse outcomes in terms of mortality that are associated with FMR, which is a ventricular disease.
Ring annuloplasty was performed in 95% of patients with myxomatous mitral regurgitation. Exceptions included severe endocarditis, where a prosthetic device is contraindicated in an infected operative field. The most common building blocks of the repair were posterior leaflet resection and commissuroplasty. The recurrence rate was very low. The freedom from reoperation was approximately 90% at ten years and about 80% at 20 years. If the posterior leaflet was repaired only, then freedom from reoperation was slightly lower, while if there was true anterior leaflet pathology the reoperation rate was slightly higher. This is comparable to the results from other groups throughout the world.
All 236 patients with FMR had ring annuloplasty done. Additionally, 77% had a concomitant coronary artery bypass grafting. Freedom from reoperation was much lower because FMR is a ventricular problem. As the left ventricle expands further after mitral valve repair, the patient may develop recurrence of mitral regurgitation even if a very small annuloplasty ring is used.
In patients with rheumatic mitral valve disease, repairs were mainly based on commissuroplasty and subvalvular extension. In this group, many patients had a calcified annulus, therefore a ring was not used in the majority of patients. The freedom from reoperation in this group was not good. At ten years it was 66%, at 20 years 34%, and at 30 years, only 10% did not require reoperation. Overall, rheumatic fever leading to mitral regurgitation is a challenging problem and older patients ought to be considered for primary mitral valve replacement.
Together, these data show that the etiology of mitral valve regurgitation determines the outcome of mitral valve repair (Table 1).
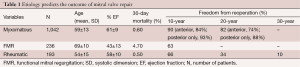
Full table
Minimally invasive mitral valve repair
Minimally invasive mitral valve repair is an area of increasing interest. This is because minimally invasive mitral valve surgery was found to decrease trauma, blood transfusion requirements, and costs (18,19). If done properly, it also increases patient satisfaction (20). However, it is important that minimally invasive surgery deliver the same quality of mitral valve repair. If quality is compromised, then surgeons may be accused of doing minimally invasive surgery for marketing and salesmanship.
The lower ministernotomy approach for mitral valve repair was popularized in our hospital (21). This allows one to see the mitral valve in the usual view through a much smaller and cosmetically pleasant incision. Transesophageal echocardiography is very important for minimally invasive mitral valve repair. It confirms the position of the right atrial and coronary sinus catheters, and shows deairing of the heart after mitral valve surgery. We use venous side suction, transfemoral catheters for venous and arterial aortic cannulation, as well as special vents. Our series of 707 patients with minimally invasive mitral valve repair showed very good early and late results, specifically there was an early mortality rate below 1% and a late mortality rate of about 7% (22). Moreover, in a published cohort of 358 patients with mitral valve repair, the freedom from reoperation was equivalent to open mitral valve repair at 92% after five years (23). This confirms the principle that the results of minimally invasive mitral valve surgery must be as good as the full sternotomy approach.
Robotic mitral valve repair
Robotic mitral valve repair can be performed via a small incision under the right breast. This operation can be performed with very low mortality, good results and a short length of stay (24,25). Problems may occur if surgeons doing robotic mitral valve repair have not been exposed to conventional techniques of repairing the valve and subsequently have results that are not as good as when using the conventional techniques. However, in the proper hands it is a very effective way of repairing the mitral valve and is as safe and effective as conventional repair (26).
Conclusions
Mitral valve repair surgery is a team effort, involving cardiac surgeons, cardiologists and cardiac anesthesiologists. A good repair record will result in early referral to valve centers for myxomatous mitral valves. High quality intra-operative transesophageal echocardiography is extremely important. Finally, experienced surgeons utilizing simplified long-lasting repair techniques will be very successful.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cutler EC, Levine SA. Cardiotomy and valvulotomy for mitral stenosis; experimental observations and clinical notes concerning an operated case with recovery. Boston Med Surg J 1923;188:1023-7.
- Ellis LB, Harken DE. Closed valvuloplasty for mitral stenosis. A twelve-year follow-up study of 1571 patients. N Engl J Med 1964;270:643-50. [PubMed]
- Lillehei CW, Gott VL, Dewall RA, et al. Surgical correction of pure mitral insufficiency by annuloplasty under direct vision. J Lancet 1957;77:446-9. [PubMed]
- Lillehei CW, Levy MJ, Bonnabeau RC Jr. Mitral valve replacement with preservation of papillary muscles and chordae tendineae. J Thorac Cardiovasc Surg 1964;47:532-43. [PubMed]
- McGoon DC. Repair of mitral insufficiency due to ruptured chordae tendineae. J Thorac Cardiovasc Surg 1960;39:357-62.
- Kirklin JW. Replacement of the mitral valve for mitral incompetence. Surgery 1972;72:827-36. [PubMed]
- Hansen DE, Cahill PD, DeCampli WM, et al. Valvular-ventricular interaction: importance of the mitral apparatus in canine left ventricular systolic performance. Circulation 1986;73:1310-20. [PubMed]
- Timek TA, Dagum P, Lai DT, et al. Will a partial posterior annuloplasty ring prevent acute ischemic mitral regurgitation? Circulation 2002;106:I33-I39. [PubMed]
- Carlhäll C, Kindberg K, Wigström L, et al. Contribution of mitral annular dynamics to LV diastolic filling with alteration in preload and inotropic state. Am J Physiol Heart Circ Physiol 2007;293:H1473-9. [PubMed]
- Cohen LH, Koster JK, Mee RB, et al. Long-term follow-up of the Hancock bioprosthetic heart valve: a 6-year review. Circulation 1979;60:87-92. [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-142. [PubMed]
- Duran CG, Pomar JL, Revuelta JM, et al. Conservative operation for mitral insufficiency: critical analysis supported by postoperative hemodynamic studies of 72 patients. J Thorac Cardiovasc Surg 1980;79:326-37. [PubMed]
- Cohn LH, Couper GS, Aranki SF, et al. Long-term results of mitral valve reconstruction for regurgitation of the myxomatous mitral valve. J Thorac Cardiovasc Surg 1994;107:143-50; discussion 150-1. [PubMed]
- Brinster DR, Unic D, D'Ambra MN, et al. Midterm results of the edge-to-edge technique for complex mitral valve repair. Ann Thorac Surg 2006;81:1612-7. [PubMed]
- Gillinov AM, Cosgrove DM 3rd, Wahi S, et al. Is anterior leaflet repair always necessary in repair of bileaflet mitral valve prolapse? Ann Thorac Surg 1999;68:820-3; discussion 824. [PubMed]
- DiBardino DJ, ElBardissi AW, McClure RS, et al. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010;139:76-83; discussion 83-4. [PubMed]
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34. [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Cohn L. Operative incisions for minimally invasive cardiac surgery. Op Tech Thoracic Cardiovasc Surg 2000;5:146-55.
- McClure RS, Cohn LH, Wiegerinck E, et al. Early and late outcomes in minimally invasive mitral valve repair: an eleven-year experience in 707 patients. J Thorac Cardiovasc Surg 2009;137:70-5. [PubMed]
- Greelish JP, Cohn LH, Leacche M, et al. Minimally invasive mitral valve repair suggests earlier operations for mitral valve disease. J Thorac Cardiovasc Surg 2003;126:365-71; discussion 371-3. [PubMed]
- Nifong LW, Chitwood WR, Pappas PS, et al. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg 2005;129:1395-404. [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.





