Robotic surgery for posterior mediastinal pathology
Clinical vignette
A 51-year-old Caucasian female presented for evaluation of a superior posterior mediastinal mass. The lesion was incidentally found after the patient was involved in a motor vehicle collision. During her trauma evaluation, a computed tomography scan of the chest showed a left paraspinal lesion measuring 2.4 cm by 2 cm (Figure 1). The Hounsfield units were consistent with fluid versus low density soft tissue mass.
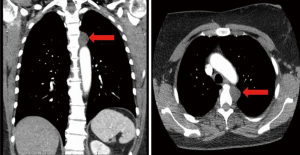
She had a past medical history consisting of arthritis, fibromyalgia and subarachnoid hemorrhage related to her accident. Her previous surgical procedures included cesarean section, total abdominal hysterectomy with salpingo-oophorectomy and left arm open reduction with internal fixation. She smoked half a pack of cigarettes daily, having done so for her entire adult life. She denied any limitation to her daily activities and had a good performance status. She worked as a registered nurse.
The patient was consented for a left robotic excision of mediastinal mass.
Surgical techniques
Preparation
The patient is initially placed supine on the operating room table (Video 1). Single lumen endotracheal intubation is first performed after induction with general anesthesia. Fiberoptic bronchoscopy is then carried out to confirm no endobronchial involvement of the mass. A left-sided double lumen endotracheal tube is then placed.

Patient positioning for robotic resection of a left-sided posterior mediastinal mass requires a right lateral decubitus position as opposed to the modified supine position typically used for thymectomy (1). At our institution, this is carried out with diffuse padding of pressure points. We do not use a bean bag for stability, nor do we use an axillary roll. The patient is secured to the operating table with safety straps and cloth tape.
Exposition
The positioning of the robot in relation to the patient is a crucial part of surgical planning and is dependent on the location of the mediastinal pathology. For lesions (anterior or posterior) above the inferior pulmonary vein, we use the standard completely portal robotic lobectomy with four arms (CPRL-4) (2,3). In this setting, the robotic docking system is placed over the patient’s head in a cephalad position. For mediastinal pathology inferior to the inferior pulmonary vein, a different approach is used. In this situation, the robot approaches from the back of the patient thus the target lesion should be located between the arms of the robot and the robot itself (2). In this position the robotic ports are placed in a line from cranial to caudal, anterior to the axilla. This positioning allows for optimal visualization of the diaphragm and lesions low in the posterior mediastinum (2).
In this patient, the CPRL-4 method was used, placing all ports over the left seventh rib (1,2,4).
Operation
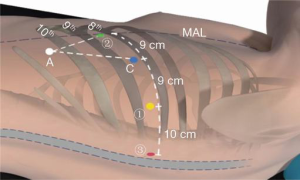
The position for the robotic ports is marked on the skin along the eighth intercostal space, as noted in Figure 2. Robotic arm No.3 is located 3 cm from the spine (most posterior), robotic arm No.1 (right arm) is 9 cm from robotic arm No.3, the camera port is 9 cm from robotic arm No.1, and robotic arm No.2 is 9 cm from robotic arm No.1 (most anterior). An assistant port is triangulated behind robotic arm No.2 (the left arm) and the camera port, as low in the chest as possible without disrupting the diaphragm. The camera port is placed initially, which permits carbon dioxide insufflation of the left chest to 15 mmHg. Once all ports are placed, the robot is docked. A cadiere instrument is used for the left robotic arm and a curved bipolar dissector is used for the right robotic arm. A thoracic grasper is used for robotic arm #3, and can assist with retraction of the lung or the mass.
Any pneumolysis is then carried out to free the lung from the chest wall. The pleura is then incised using the thoracic bipolar grasper. The mass is then dissected off of the superior portion of the aortic arch. Special care is made to use the bipolar device to cauterize all small arterial branches coming off the aorta. We are also careful to clearly identify the sympathetic chain and avoid injury to this structure. In cases where the posterior mediastinal mass is closer to the airway, thermal injury to the membranous section of the mainstem bronchi should be carefully avoided. In this case, the mass was noted to be cystic in nature. We were able to dissect the lesion without rupture of the mass. The mass was then removed from the chest with an intracorporeal bag.
Completion
A 19 French Blake drain was placed, connected to suction. The four incisions were closed in two layers, with 2-0 vicryl stitches for the dermis and 3-0 vicryl stitches for the subcuticular layer. The drain was removed and the patient was discharged home on postoperative day one.
Comments
Clinical results
Our institution has had good results using the above method. From 2009 to 2011, 75 patients underwent robotic operations for posterior mediastinal pathology (2). Of these, 11 were for masses and 11 were for cysts; the remainder were for lymphadenopathy, esophageal or diaphragmatic pathologies. The only patients in the series who experienced major morbidity or conversion to thoracotomy were those with epiphrenic diverticula. Median operative time was 95 minutes and decreased progressively with experience, and median docking time for the robot was 12 minutes for the last 55 cases. The median length of hospital stay was one day.
Advantages
Pursuing a robotic approach for posterior mediastinal tumors has many advantages for the surgeon. The enhanced view via the stereoscopic three-dimensional camera offers magnified visualization for accurate, fine dissection. Robotic surgery also places camera manipulation in the hands of the surgeon. We have also found that the wristed instruments with multiple degrees of movement are ideal for carefully dissecting out and cauterizing the occasional vascularized attachments to posterior mediastinal lesions. Our technique also allows for insufflation of the intra-thoracic cavity which safely compresses the lung parenchyma and depresses the diaphragm, allowing for greater working space (5).
Caveats
We have found that successful robotic resection of posterior mediastinal lesions is dependent on preoperative planning and proper port placement/robotic docking techniques. As noted above, lesions that are above the level of the inferior pulmonary vein are targeted well with standard CPRL-4 port placement over the seventh rib, while lesions below the vein are better approached with ports arrayed in a cranial-to-caudal orientation. Suboptimal port placement can lead to frustration and difficulty. Other issues related to robotic thoracic resections include the cost related to acquisition and utilization of the robot. This requires an institution dedicated to pursuing and growing a successful robotic program (2). Finally, the advantages of robotic approaches to posterior mediastinal lesions compared to video-assisted thoracoscopic surgery in terms of patient outcomes are not well established.
Conclusions
In conclusion, we have found that robotic resection of posterior mediastinal pathology is safe and effective. Utilizing the robot for these types of resections can prove to be efficient with proper team training and communication as well as preoperative planning for ideal port placement.
Acknowledgements
R.J.C has a financial disclosure with Intuitive Surgical.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7.
- Minnich DJ, Bryant AS, Cerfolio RJ. Thoracoscopic and robotic dissection of mediastinal lymph nodes. Thorac Surg Clin 2012;22:215-8. [PubMed]





