Video-assisted thoracoscopic pneumonectomy: The Edinburgh posterior approach
Introduction
Twenty years of experience in Video-assisted thoracoscopic lung surgery (VATS), have led to the development of innovative techniques to rival open thoracotomy for major lung resections (1,2). Acceptability of the technique relates to benefits including; reduced length of hospital stay, decreased blood loss, decreased pain, improved cosmesis, earlier return to normal activities and improved tolerance of chemotherapy (3-6). Whilst the technique was initially limited to pulmonary lobectomy, centers are now routinely performing VATS pneumonectomy. Whilst it remains unclear as to whether the technique affects survival, VATS pneumonectomy provides a safe alternative to open resection, which confers many of the benefits outlined above (7,8). An additional plus, is that patients are not subject to postoperative air leaks, which are one of the major complications of VATS lobectomy, often resulting in prolonged hospital stays and increased morbidity and mortality (9,10).
The technique of VATS pneumonectomy described here is that which is currently employed in the unit of cardiothoracic surgery at the Royal Infirmary of Edinburgh.
Clinical summary
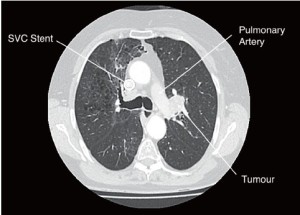
A 70-year-old lady presented with an incidental finding of a left upper lobe lesion on plain chest X-ray, during investigation of probable chronic obstructive pulmonary disease. Past medical history included a right upper lobe, small cell lung cancer in 2002, successfully treated with chemo/radiotherapy. Co-morbidities were emphysema, a transient ischemic attack and at the time of surgery the patient had a SVC stent in-situ for SVC obstruction.
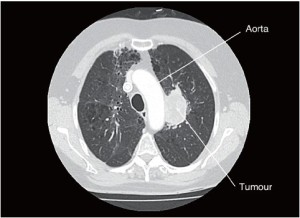
PET CT imaging revealed a 50 mm AP × 30 mm TR, T2bN0M0 lesion in the left upper lobe which may be adherent to the aorta and pulmonary artery (Figures 1,2). Bronchoscopy demonstrated an intraluminal lesion obstructing the left upper lobe bronchus orifice and CT guided biopsy returned a diagnosis of Non-Small Cell Lung Cancer. Lung function was assessed via formal spirometry with a FEV1 of 1.85 (105% predicted), a FVC of 2.6 (112% predicted) and an FEV1/FVC ratio of 72% (Video 1).
Pre-operative assessment
Pneumonectomy is associated with high morbidity and mortality (11). Patient selection is therefore paramount and pre-operative assessment is perhaps the most crucial stage of the process. Operator experience is also a key factor and individuals unfamiliar with VATS techniques should not attempt VATS pneumonectomy.
Whilst lung conservation is a primary aim of surgery, anatomical considerations often preclude suitability for lobar and sub-lobar resections. In patients where broncho-vascular reconstruction is not viable, VATS pneumonectomy is the procedure of choice in our unit for individuals in whom there is low bulk hilar involvement.
Pre-operative assessment, anaesthesia and positioning is the same as for VATS lobectomy as described by Richards et al., with the key points summarized here (12). Pulmonary function is assessed via a combination of spirometry and CO2 transfers in all surgical candidates. Whilst providing an estimate of predicted lung function following resection, this also gives a useful baseline value for post-operative comparison and predicts tolerance to double-lumen intubation. In individuals in whom there is a theoretical risk of pulmonary hypertension, Echo assessment of pulmonary artery pressure is undertaken prior to surgery. Further assessment is performed depending on existing co-morbidities.
As part of the staging process, all surgical candidates undergo positron emission tomography-CT (PET-CT) with 18F-fluordeoxyglucose (18F-FDG) subsequent to routine imaging. Regardless of the outcome however, we currently perform mediastinoscopy on all patients due to a 5% false negative rate for detection of lymph node metastases with PET-CT (13). The sampling of lymph node stations is performed in accordance with current guidelines from the European Society of Thoracic Surgeons.
Anaesthesia and positioning
The patient is placed in the lateral decubitus position with the arms extended to 90o and the elbows flexed to 90o. To protect the intercostal neurovascular bundles the table is “broken” or flexed to maximise the intercostal spaces.
General anaesthesia is induced and intubation is achieved via a double lumen endotracheal tube, which allows independent ventilation of either lung. This permits the lung on the operative side to be deflated whist ventilation is maintained to the contralateral lung. Intercostal nerve blocks are utilized for peri-operative analgesia rather than of epidural analgesia to avoid the risk of phrenic nerve involvement and hypotension.
Technique
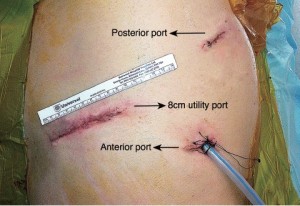
Three VATS ports are created to facilitate optimal views of the posterior hilum and placement of instruments (Figure 3). For VATS pneumonectomy it is necessary to use a slightly extended utility port to permit extraction of the entire lung. A 5-6 cm incision (which can be extended later if necessary) is made in the 7th intercostal space adjacent to the anterior border of latissimus dorsi. A zero degree 10 mm high definition video thoracoscope is temporarily placed through this port to allow safe completion of the anterior and posterior port sites. The posterior incision (approx. 1.5 cm) is made in the auscultatory triangle at the point nearest to the upper end of the oblique fissure. A final 2 cm incision is made in the mid axilliary line in a horizontal plane corresponding to the upper third of the utility port.
The first step in the procedure is to confirm resectabilty and identify invasion of the chest wall, pleurae and hilar structures including the aorta, pulmonary artery and bronchus. The posterior approach allows excellent visualization of the posterior hilum and the structures mentioned above. It is also beneficial in assessing the hilar lymph nodes adjacent to the bronchus.
Dissection is commenced in the posterior hilum with a combination of blunt and sharp incision of the mediastinal pleura, overlying the descending aorta. Any adherent vessels are identified, clipped and cut. At this stage in the procedure care must be taken to avoid inadvertent injury to the vagus, phrenic and recurrent laryngeal nerves. It may however be necessary to deliberately sacrifice these depending on tumour spread. The pleura is reflected with a blunt instrument and a Roberts is passed anteriorly to the main bronchus taking care not to injure the pulmonary artery which lies directly anterior. A vascular sling is passed into the jaws of the Roberts which permits gentle retraction. During this manoeuvre, better visualization of hilar lymph nodes, (5&7 demonstrated in the video here) is enabled. Excision of these lymph node packets at this stage will facilitate exposure of the sub-carinal region and the proximal bronchus, and frozen section can be requested if deemed necessary.
Attention is now turned to the inferior and anterior hilum, with the inferior pulmonary ligament divided first. This allows exposure of the inferior and superior pulmonary veins and the pulmonary artery sequentially. When exposure is adequate blunt dissection is used to skeletonize the pulmonary veins.
At this stage consideration is given to the order of division of the hilar structures. Experience tells us that the order of division does not affect outcome and therefore safety should be a priority during this part of the operation.
In the case presented here the superior pulmonary vein was divided using a tan 45 mm tri-stapler passed through the posterior port. Leaving the inferior pulmonary vein at this stage can prevent vascular engorgement, which may hinder progress. Attention was then turned to the bronchus. The key manoeuvre here is to apply gentle retraction to the vascular sling permitting adequate exposure of the bronchus at the hilum. This will allow the bronchus to be taken as close to the carina as possible. Ensuring this is achieved will reduce the risk of broncho-pleural fistula post-operatively. This is accomplished with a purple 45 mm tri-stapler passed through the anterior port. Division of the bronchus allows direct visualization of the pulmonary artery, which is taken with a tan 45 mm tri-stapler. The final vessel remaining is the inferior pulmonary vein. This is taken with a tan 45 mm tri-stapler passed through the anterior port.
Following division of the hilar structures the specimen is retrieved through the utility port. A plastic bag is passed through the utility incision to prevent contamination and tumour seeding in the wound. A paravertebral catheter is placed adjacent to the sympathetic chain through which local anaesthetic can be administered. Finally, a 32F chest drain is placed through the anterior port prior to closure of the port sites.
Post-operative management
All patients undergo a routine post-operative chest X-ray whilst in the recovery room. A naso-gastric tube is passed whilst the patient is still sedated. The patient is kept nil by mouth with a fluid restriction of 1.5 L. These measures help to prevent aspiration and development of post-pneumonectomy pulmonary oedema. Analgesia, antibiotics and anti-coagulation are administered routinely in accordance with local guidelines.
Comments
Current debate exists as to whether thoracoscopic pulmonary resections are more effectively performed via an anterior or posterior approach. Based on experience from over 800 VATS major lung resections the preference of the authors is the posterior approach. This technique permits superior views of the posterior hilum, which is crucial during pneumonectomy when dissecting and dividing the main bronchus and major pulmonary vessels. As reported by Richards et al., this approach also enhances the view of the mediastinal node packets which facilitates lymphadenectomy (13). Additionally, the positioning of the camera in the posterior approach allows direct visualization of the instrument tips which is essential for safe dissection.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Walker WS, Carnochan FM, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2.
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402.
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70.
- Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2006;132:507-12.
- Sugiura H, Morikawa T, Kaji M, et al. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 1999;9:403-8.
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7.
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6.
- Sahai RK, Nwogu CE, Yendamuri S, et al. Is thoracoscopic pneumonectomy safe? Ann Thorac Surg 2009;88:1086-92.
- Craig SR, Walker WS. Initial experience of video assisted thoracoscopic pneumonectomy. Thorax 1995;50:392-5.
- Rice TW, Okereke IC, Blackstone EH. Persistent air-leak following pulmonary resection. Chest Surg Clin N Am 2002;12:529-39.
- Miller DL, Deschamps C, Jenkins GD, et al. Completion pneumonectomy: factors affecting operative mortality and cardiopulmonary morbidity. Ann Thorac Surg 2002;74:876-83; discussion 883-4.
- Richards JM, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: The Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:61-9.
- Carnochan FM, Walker WS. Positron emission tomography may underestimate the extent of thoracic disease in lung cancer patients. Eur J Cardiothorac Surg 2009;35:781-4; discussion 784-5.