How to set up a successful TAVI program
Introduction
The introduction of transcatheter aortic valve implantation (TAVI) has revolutionized the management of aortic valve disease in the elderly and high-risk population. Experiences and results around the World have varied widely and the importance of a multidisciplinary and cohesive heart team is mandatory in this challenging group of patients. Our Unit started a TAVI program (Edwards Sapien transfemoral and trans-apical) some three years ago. We have now performed 70 procedures, with no procedural deaths and only three thirty day deaths, none of which were cardiac related. Some of this was good luck, but a lot of it was good management and a credit to the wider team involved. The aim of this paper is to offer some insight into the successful implementation of a TAVI program into the treatment armamentarium of a cardiovascular unit.
Pursuing a TAVI program-a decision not to be taken lightly
TAVI is a highly resource and labor intensive procedure. The impact on other procedures and patients must be considered before embarking on a TAVI program. Intensive care unit pressures and effects on elective cardiac surgical procedures are especially important. The waiting list times for younger cardiac surgical patients may increase and there may be morbidity and mortality associated with this.
However you may like to analyze it - TAVI is not cheap. There are multiple health economic models that can be used to justify TAVI, but there is no getting away from the fact that the prostheses are around $30,000 (AUD) and there are significant procedural related costs. The prostheses are not currently reimbursable under private health insurance schemes (in Australia) and the institution must bear the cost. A recent economic analysis of the Partner One Trial medically managed patients showed an almost doubling of costs in the Cohort B (TAVI) patients ($100,000 vs. $50,000) with a cost of $50,000 (USD) per life year extended for the TAVI cohort (1). These are philosophical as well as economic issues and must be weighed up when embarking on a TAVI program.
There must be quarantined time for TAVI and this needs to be arranged well in advance. Maintaining communication between the Operating Room, Angiography Suite and Intensive Care to ensure bed availability and appropriate staff are rostered for the procedure is especially important. The procedure is ideally performed in a hybrid operating suite, however it is possible to use an angiography suite. It is imperative that the angiography suite has sufficient space to accommodate a surgical setup and an ECMO circuit (our preference over a full bypass machine). It is also important to have the ability to perform rescue surgery, prior to transfer to the operating room, within the confines of the angiography suite should the need arise.
Obtaining permission-ethical and financial
Prior to commencing a TAVI program, it is a requirement in most institutions to have the procedure approved by The Ethics Committee, The New Device and Procedures Committee and by The Hospital Administration (who control the finances). We recommend personal presentations to these committees after submitting carefully written applications.
It’s then important to educate and inform the wider medical community on the benefits of TAVI and your group’s intention to pursue the therapy. Presentations involving surgeons and physicians at referring hospitals clinical meetings, to GP groups and a judicious use of the media to advertise the technology is important. Having a single point of referral is also important to make it easy for referring physicians and patients alike.
The maintenance of a database to track and report results is extremely important. This should involve contribution to national and international registries. Data collection and management needs to be factored into the program from the outset.
Training and proctoring
The two companies (Edwards Lifesciences and Medtronic) involved in the current commercially available TAVI technology are strict in their adherence to a structured training and proctoring algorithm for the teams involved in the delivery of TAVI. Several centers around the World have high volume TAVI workloads and provide a training and subsequent proctoring service (our Unit now provides proctoring for trans-apical TAVI).
Training involves lecture and laboratory based education followed by live case observations. A number of proctored cases are then completed before the site is “signed-off” (usually 8-10 cases). The company involved usually allocates the training site and the subsequent proctors. However, it is important that there is a good synergy between the visiting and host teams. Advice and mentorship for the discussion of difficult cases in the initial post-proctored period is also valuable. It’s important that there is not too much delay between the training period and the commencement of the TAVI implants.
The high-risk heart valve team-patient selection (and rejection)
The concept and importance of the “Heart Team” is no more evident than in a TAVI program. The units that have a successful TAVI program have a high level of cohesion between all parties involved, from the referring physicians through to the procedural specialists. The Heart Team includes an interventional cardiologist, a non-interventional cardiologist, a cardiac surgeon, an anesthetist, an intensive care specialist and a geriatrician. An expert opinion from a vascular surgeon is useful in borderline peripheral access cases and for peripheral access rescue surgery should the need arise. TAVI is a procedure that requires multiple skill sets and it is often unpredictable when there will be significant complications that require the immediate input of all members.
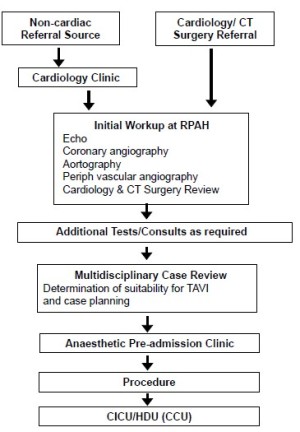
Patient selection and rejection can be challenging and is as much art as science. There have been several attempts to quantify “frailty” in the cardiac surgical patient, but much of this is derived from the geriatric literature and can be difficult to apply. All patients need to be assessed and investigated before being discussed at the high-risk heart valve multidisciplinary team meeting (MDT) (Figure 1). In our experience a higher number of patients are rejected rather than accepted for TAVI.
We use balloon aortic valvuloplasty (BAV) liberally for several reasons. Firstly, it is a useful discriminator between shortness of breath from respiratory causes ie. it doesn’t improve the symptoms, and patients who improve dramatically with temporary relief of their aortic stenosis. BAV is also a useful bridge to definitive therapy, which allows a planned procedure and time to address other issues (coronary and carotid stenting as an example) and to allow for the considerable (up to six months) waiting time for the procedure.
Some patients should be offered open AVR surgery, especially if they are medically fit and have concomitant cardiac issues such as coronary artery or mitral valve disease. Even more importantly, some patients should be offered medical therapy only, as the risk of TAVI may outweigh the benefit or the co-morbidities (particularly respiratory) may negate any benefit in improvement of symptoms that TAVI may confer. Indeed, many patients are referred and have an expectation (as does the referring physician) that “something” will be done, and it can be very difficult to manage patients and their families through a decision that no procedure can be offered.
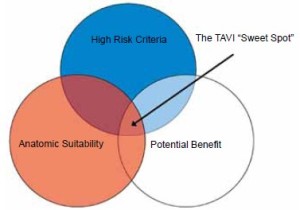
The PARTNER 1 (2) and PARTNER 2 (3) trials were important studies in the defining of patients that are appropriate for TAVI. Firstly, the PARTNER 1 patients were considered too high risk for open AVR and the higher risk patients (logistic EUROSCORE >30) had a very poor long-term survival, questioning the value (financial and affects on other resources) of the procedure in this cohort. The PARTNER 2 patients (potential surgical candidates) had equivalent results between open AVR and TAVI, with significantly less paravalvular leak in the open AVR group, suggesting that open AVR is superior in this population. Therefore it is important to try and find the “sweet spot” for the best use of TAVI (Figure 2). We feel that this is the patient group that has significant co-morbidities that will make recovery after open AVR onerous, but still have a reasonable life expectancy if the aortic valve disease is corrected.
Preparation, planning and conducting the procedure
The patients are discussed at the MDT meeting and are reviewed by a surgeon, an interventional cardiologist and an anesthetist prior to proceeding. The decision on transfemoral versus trans-apical is based upon the review of angiography and CT-angiography. Small, calcified femoral or iliac vessels, a tortuous or transverse aorta are potential hazards for the trans-femoral approach. Having a successful trans-apical program has given our patients a viable alternative to the trans-femoral route and kept peripheral vascular injury to a minimum.
It is imperative that the Heart Team, the patient and the patient’s family are aware of the limit to the extent of surgery that will be offered in the event of a significant complication. For example, we feel that redo sternotomy and repair of a type A dissection in a nonagenarian is inappropriate and would not be undertaken. However, an 80 year old with no previous history of cardiac surgery, having TAVI for issues such as poor respiratory reserve would be offered rescue AVR or other surgery should the need arise. This is discussed at length with all parties and then documented in the patient’s file prior to the procedure. All patients are offered temporary ECMO support, which will be discussed in more detail below.
A surgeon and cardiologist are present and scrubbed for each procedure. For the trans-femoral cases a second cardiologist is also present and for the trans-apical procedures a second surgeon is present. Patients are prepped as they would be for routine open heart surgery in our unit. All patients are intubated, have usual cardiac surgical monitoring including pulmonary artery catheter, radial arterial line, indwelling urinary catheter and a trans-oesophageal echo. All patients have external defibrillator pads and are prepped and draped to allow access for either trans-femoral, trans-apical or a median sternotomy approach. The majority of femoral access is via a total percutaneous approach with vessel closure via previously deployed Proglide Perclose devices. Vessels are imaged with a Sonosite ultrasound or using digital subtraction angiography for central, infra-inguinal ligament arterial puncture. A 6 French catheter is placed in the femoral artery for later insertion of the TAVI introducer and the femoral vein for placement of the pacing wire. Two 6 French catheters are placed in the contralateral femoral vessels in all patients for V-A ECMO if required. The contralateral arterial sheath can also be used for the aortogram pigtail catheter.
V-A ECMO-strategies and limitations
Serendipitously the HIN-1 epidemic and our extensive use of V-V ECMO preceded the commencement of our TAVI programme by six months. This coincided with dramatic improvements in cannula technology, miniaturization and portability of ECMO pumps. The expertise and level of familiarity with ECMO within the Unit has been extended to many uses, including high-risk catheter based procedures, including PCI and TAVI (4), cardiac arrest situations, international retrievals for respiratory and cardiac failure (5), Type IV thoraco-abdominal aneurysm surgery amongst other procedures.
We prefer ECMO to a full CPB circuit for reasons of space, reduced circuit prime, reduced ACT requirement and ease of use. V-A ECMO has been used liberally in the elective situation for patients with poor left or right ventricular function and in patients with incompletely treated coronary artery disease. It has been used emergently for acute right heart failure, decompressing the heart to deal with left ventricular apex haemorrhage and to facilitate rescue procedures such as valve-in-valve sealing of aortic root rupture, managing valve embolisation and thoracotomy for decompression of tamponade. If Proglides haven’t been predeployed than the arterial puncture site needs to be managed with a surgical cut-down and purse-string repair, whilst the venous site is managed with a large percutaneous pursestring that is changed to interrupted Prolene sutures after Protamine administration.
The V-A ECMO cannulae are usually placed percutaneously, after predeploying Proglide sutures, a technique pioneered by our Unit (6). If peripheral arterial access is poor then a right axillary artery cut-down is performed with a Seldinger technique cannulation after placing 5/0 Prolene purse-strings.
All patients have reperfusion and rest on ECMO or transfer to an operating theatre for rescue surgery if that is deemed appropriate before undertaking TAVI (as discussed previously discussed). We have made a unilateral decision that no patient will be offered long-term ECMO support in the Intensive Care Unit. We feel that the chance of survival after this level of support in this elderly and frail population is virtually nil and is inappropriate.
After care in the Intensive and Coronary Care Units
As with the preoperative planning and conduct of the procedure, the after care is managed as we would normally manage major open heart surgical patients. All patients are returned to the Intensive Care Unit intubated, with extubation performed after normothermia, appropriate gas exchange and level of consciousness is obtained. It is important to quell any nihilism when it comes to elderly and frail patients and utilise all manner of support necessary in the acute setting. This includes early and liberal use of temporary haemodialysis, aggressive correction of respiratory issues and early use of broad-spectrum antibiotics as sepsis is often masked in the elderly. Permanent pacemakers, if required, are also placed early, allowing removal of temporary trans-venous wires to facilitate mobilization.
Patients are discharged from the Intensive Care Unit to the Coronary Care Unit where aggressive medical management and physiotherapy is continued. The patients are discharged to the ward (medical or surgical) when the level of medical care required is appropriate.
Conclusions
We commend the pursuit of TAVI to all clinicians with a high level of interest in high-risk and elderly patients with cardiovascular disease. A TAVI program is highly rewarding for patients and clinicians alike. The results can be spectacular, restoring a high quality of life to elderly and frail patients with hitherto untreatable aortic valve disease. However, TAVI requires a high level of co-operation between multiple personnel across multiple departments and it is these units that have the highest chance of having a successful TAVI program.
Acknowledgements
The authors wish to thank Dr Lisa Simmons, Dr Brian Bailey, Dr Scott Murray and A/Prof Paul Forrest for their continued commitment to the TAVI program.
Disclosure: Dr Michael Wilson is a paid trans-apical TAVI proctor for Edwards Lifesciences. There are no other disclosures or conflicts of interest.
References
- Reynolds MR, Magnuson EA, Wang K, et al. Costeffectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation 2012;125:1102-9.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98.
- Spina R, Forrest AP, Adams MR, et al. Veno-Arterial Extracorporeal Membrane Oxygenation for High-Risk Cardiac Catheterisation Procedures. Heart Lung Circ 2010;19:736-41.
- Forrest P, Cheong JY, Vallely MP, et al. International retrieval of adults on extracorporeal membrane oxygenation support. Anaesth Intensive Care 2011;39:1082-5.
- Ramponi F, Yan TD, Vallely MP, et al. Total percutaneous cardiopulmonary bypass with Perclose ProGlide. Interact Cardiovasc Thorac Surg 2011;13:86-8.