Patient selection for transcatheter aortic valve implantation: An interventional cardiology perspective
Transcatheter aortic valve implantation (TAVI) has emerged as a highly effective minimally invasive treatment symptomatic for severe calcific aortic stenosis in patients at high or prohibitive surgical risk. The success of TAVI has been determined by a number of factors, but in particular by appropriate patient selection. Appropriate patient selection involves identifying patients with the potential to benefit most from TAVI and individualizing the bioprosthesis type and size, and the vascular access site for each case. We present herein, our critical appraisal on patient selection for TAVI: an interventional cardiology perspective.
Key words: Aortic stenosis; transcatheter heart valve; transcatheter aortic valve implantation; TAVI; Edwards SAPIEN; Medtronic CoreValve; patient selection
Introduction
In April 2002, Alain Cribier performed the first-in-human transcatheter aortic valve implantation (TAVI). In the ensuing decade, this novel technique has evolved into a relatively mature widely accepted treatment for high or prohibitive surgical risk patients with symptomatic severe calcific aortic stenosis (AS) requiring aortic valve replacement (AVR). The Edwards SAPIEN transcatheter heart valve (THV) (Edwards LifeSciences, Irvine, CA) (Figure 1) and Medtronic CoreValve (Medtronic, Minneapolis, MN) (Figure 2) are licensed in Europe for implantation in selected patients, and in excess of 80,000 patients worldwide have undergone TAVI.

Careful, considered patient selection by a team of experienced interventional cardiologists, cardiac surgeons, anaesthetists, and imaging specialists (the heart team) has been at the core of the TAVI success story (1). Patient selection for TAVI continues to evolve however, as the almost daily publication of TAVI-related data defines and refines the patient, anatomical, and procedural factors that determine successful implantation. Put simply, appropriate patient selection implies identifying candidates who benefit most from TAVI, however, this can be a complex process (Table 1).
| Table 1 Pre-procedure screening recommendations | |||
| Laboratory indices |
Full blood count, serum urea, creatinine and electrolytes, C-reactive protein, serum transaminases, serum albumin, coagulation profile, blood culture, sputum culture, mid-stream urine, glycosylated haemoglobin, human immunodeficiency virus, hepatitis serology |
||
| Clinical data to calculate logistic EuroSCORE or STS score | Detailed clinical history, examination and current medication list, 12 lead electrocardiography, echocardiography (transthoracic/transoesophageal), coronary angiography, peripheral vascular screening (contrast angiography/multidetector computed tomography), pulmonary function testing, right heart catheterization | ||
| Clinical parameters of comorbid conditions | Pulmonary function tests, carotid, vertebral and abdominal ultrasonography | ||
| Fragility and cognitive function* | Grip strength, graded exercise testing, walk test, physical activity level, mini-mental score | ||
| Confirmation of aortic stenosis severity and assessment of associated pathology | Echocardiography (transthoracic/transesophageal), exercise stress testing, stress echocardiography | ||
| Procedural planning | Multidetector computed tomography/transoesophageal echocardiography |
||
| Legend: STS = Society of Thoracic Surgeons; * = Elements of the fried frailty index | |||
TAVI eligibility
Potential TAVI recipients must satisfy three essential criteria in order to be deemed “TAVI-eligible” (2,3): severe symptomatic AS, high or prohibitive surgical risk, and absence of contraindications to TAVI.
Confirmation of the severity of aortic stenosis
Transcatheter aortic valve implantation is indicated for selected patients with severe AS, thus confirmation of the AS severity is mandatory in all cases. Echocardiography is the gold standard method to assess AS, and yields both important anatomic and haemodynamic information. Doppler evaluation of the peak and mean transaortic gradients and determination of the aortic valve area (AVA) by the continuity equation are recommended. Current societal guidelines define severe AS as a mean aortic valve gradient of ≥40 mmHg or an AVA of ≤1 cm2 (<0.6 cm2/m2) (3,4). In patients with low transaortic gradients, despite an AVA consistent with severe AS, dobutamine stress echocardiography is recommended to distinguish between severe and pseudosevere AS (5). The presence of symptoms is used to guide management of AS patients, however determining the nature of symptoms is not always straightforward. In cases of equivocal symptoms, exercise stress testing, and in particular stress echocardiography are advised (6).
Surgical risk eligibility
Transcatheter aortic valve implantation is indicated for selected patients at high or prohibitive surgical risk. Thus, the advent of TAVI has renewed interest in the use of surgical risk algorithms. Surgical risk has been quantified using the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) (7) and the Society of Thoracic Surgeons (STS) Predicted Risk of Mortality score (8). However, these scores share important limitations in high-risk patient subsets, most notably a limited predictive capacity and an inability to capture significant comorbid conditions in what is a heterogeneous patient group. The logistic EuroSCORE for example, has a low discriminatory power in TAVI patients (C statistics 0.61 to 0.64) (9). As such, the applicability of these scores in patient selection for TAVI has been questioned (10-12). Despite these limitations, patient enrolment in TAVI trials has been determined by a EuroSCORE >15% or an STS score >10% (13-15). However, a number of comorbid illnesses associated with adverse surgical outcomes are not included in these risk calculation scores, including: chronic lung disease [forced expiratory volume in 1 second (FEV1) <1 litre]; liver cirrhosis (Child class A or B); pulmonary hypertension (pulmonary artery systolic pressure >60 mmHg); previous cardiac surgery; porcelain aorta; recurrent pulmonary emboli; right ventricular failure; contraindication to traditional open chest surgery (wide beam radiotherapy); or cachexia (body mass index <18 kg/m2).
As such, we recommend these scores be used as a guide for patient selection, though they should not supersede clinical judgement.
Anatomical eligibility
Transcatheter aortic valve implantation is indicated for patients with severe AS who meet certain anatomical criteria, and the bioprosthesis type, size and mode of delivery are entirely reliant on pre-procedural anatomical screening. Multimodality imaging using echocardiography, mult idetector computed tomography (MDCT) and fluoroscopy/angiography are used for detailed anatomical screening (16).
Vascular screening
Evaluation of the peripheral vasculature necessitates assessment of 3 important features: the size (minimal luminal diameter), tortuosity, and calcification of the iliofemoral arteries. Vascular assessment is most commonly performed using contrast angiography or MDCT (17).
Using contrast angiography, a SFAR ratio ≥1.05 (outer Sheath diameter to Femoral Artery minimal luminal diameter Ratio) has been identified as a predictor of valve academic research consortium (VARC) major vascular complications and 30-day mortalit y (18). This ratio decreases to 1.00 in non-calcified vessels and increases to 1.10 in the presence of moderate to severe calcification.
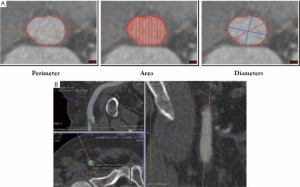
MDCT is probably the gold standard test for screening the peripheral vasculature of potential TAVI recipients (Figure 3) (19). With MDCT, assessment of vessel tortuosity, calcification, and vessel size, is enhanced compared to contrast angiography; MDCT, however, is associated with increased iodinated contrast exposure. High-pitch spiral dual source CT with minimized contrast volume may overcome this limitation (20).
Annulus assessment
Although not a distinct anatomic structure, the aortic valve annulus may be defined as the virtual ring formed at the junction of the basal attachment points of the aortic valve leaflets within the left ventricle (21). In this plane, the oval aortic annulus represents the transition point between the left ventricular outflow tract and the aortic root. Accurate measurement of the aortic annulus diameter is of critical importance for THV sizing and the short- and long-term success of the procedure. Valve oversizing risks catastrophic annulus rupture, while undersizing may result in valve migration or paravalvular regurgitation; which has been recognized as an independent predictor of long-term mortality (22).
Transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), MDCT, contrast aortography, and magnetic resonance imaging (MRI) can all be used to assess the annulus dimensions. Although much debate surrounds the choice of imaging modality for optimal measurement of the non-circular annulus for the purposes of TAVI, MDCT is becoming recognized as the gold standard.
Although TEE is the most practical imaging modality, 2-dimensional (D) measurements of the 3-D aortic annulus, can lead to underestimation of the true dimensions of the annulus (23). It has been suggested that echocardiographic measurement of the annulus potentially results in valve undersizing, and consequently increases the risk of paravalvular aortic regurgitation (24). A recent publication noted that 3-D TEE may provide more accurate aortic annular measurements than 2-D TEE (25), however the use of 3-D TEE is not yet endorsed by societal guidelines (26).
MDCT reconstruction of the annulus orthogonal to the center-axis of the left ventricular outflow tract allows for the assessment of minimal and maximal diameter, circumference, and area measurements. MDCT data has confirmed that the majority of aortic annuli are oval, and has shown the mean difference between the maximum and minimum diameter of the aortic annulus to be 6.5 mm (95% confidence interval, 5.7-7.2) (27). Assessment of the aortic valvar structure with MDCT also facilitates assessment of cusp morphology, and the distribution of calcification. In the light of these advantages, MDCT is becoming the default imaging modality to assess aortic annular dimensions. It remains unclear how to exactly apply MDCT-based valve sizing to existing echocardiographic sizing criteria (27) - this will be further discussed below.
Contraindications to TAVI
Although many elderly patients with severe AS meet the “inclusion” criteria for TAVI, these procedures are not suitable for all. As the technology and physician experience evolves, some init ial cont raindicat ions have been discounted, while others have emerged.
Recently, it has been recognised that frailty and futility are important concepts when selecting patients for TAVI. Frailty is considered to be a distinct clinical syndrome characterized by decreasing muscle mass, energy expenditure, and malnutrition, and imparts extreme vulnerability to adverse events (28). Futility implies that a patients’ condition is so advanced, that meaningful improvement will not be achieved despite a technically successful intervention. In this regard, the 2-year results of the Partner trials offer much food for thought. In inoperable patients (cohort B), two-year mortality following TAVI was 43.3%, the majority of whom died from cardiovascular causes (64.9%) (29). Similarly, two-year mortality was 33.9% in high operative risk patients that received TAVI in Partner cohort A (22). These data send a clear message: performing TAVI on patients who derive little long-term benefit due to irreversible coexisting conditions should be avoided, particularly in the current resource-limited environment. Preliminary analyses suggest that patients with lower surgical risk scores (EuroSCORE, STS) derive the most benefit from TAVI (29,30), though further analysis of large patient populations is required before using risk score cutoff- points to define TAVI-ineligible patients. The role of specific risk scores to assess frailty, such as the Fried Frailty Index (28), is yet to be determined.
In borderline cases, where the decision to proceed to TAVI is not clear due to advanced age, co-morbid conditions or other factors, percutaneous balloon aortic valvuloplasty (BAV) can represent a useful additional selection tool (31). BAV strategy is associated with rapid functional improvement and thus, enhances the prediction of very frail patients who might benefit from TAVI, without incurring the risk or cost associated with a full TAVI procedure. In one study, reevaluation of borderline patients 30-days after BAV, deemed 46% TAVI eligible, 28% surgical AVR eligible, while 21% demonstrated no functional improvement and were therefore assigned to medical therapy (31).
Procedural considerations
Careful planning of the TAVI procedure itself is a critical component of the patient selection process. The vascular access site and the bioprosthesis type and size are crucial to procedural success.
Vascular access
Selection of the vascular access site is based on careful pre-procedural screening and should be individualized for each patient. Our preference is to select the least invasive route possible for TAVI. As such, all patients are evaluated for the feasibility of a transfemoral approach, and an alternative approach is only selected in the setting of a prohibitively small or diseased iliofemoral arterial system, the presence of mobile plaque, excessive calcification, or extreme tortuosity of the descending thoracic aorta. Alternative approaches in our order of preference are: subclavian (32-41); transaortic (42-48); and transapical (49-52). Importantly, we do not push the limits of the available technology and if the peripheral vasculature is unfavourable, an alternative access is selected. Feasibility does not equal safety.
The femoral artery is considered to be default vascular access site for TAVI. Theoretical advantages of the transfemoral approach include avoidance of general anaesthesia, thoracotomy, incision of the apex of the left ventricle, and potential complications such as delayed wound healing. Advantages to the non-transfemoral approaches include avoidance of peripheral vascular (except subclavian) and aortic complications. Importantly, the risk of periprocedural cerebral embolization and stroke appears to be similar between these different strategies (53,54).
To date, few studies have directly compared clinical outcomes between transfemoral and non-transfemoral TAVI (14,53,55,56). Compared to the femoral approach, non-transfemoral approaches (largely transapical) tends to be performed on higher risk patients, as assessed by EuroSCORE (largely driven by peripheral arterial disease). Transfemoral TAVI is associated with an increased risk of vascular complications, while non-transfemoral procedures have a higher risk of bleeding and surgical conversion (14,53). To date, non-transfemoral TAVI has been associated with increased 30-day and two-year mortality (14,53). Although this mortality difference may be due to the more advanced risk profile of the non-transfemoral patients, it is possible that these procedures themselves confer increased risk. General anaesthesia, thoracotomy, incision of the left ventricular apex and manipulation of a large catheter within the left ventricle are not without risk. However, it must be stated that as surgical experience with these devices improves, and dedicated transapical and transaortic devices are developed, improved outcomes are emerging with non-transfemoral TAVI (57). The advent of the transaortic approach is a particularly encouraging technique that avoids many of the theoretical complications associated with transapical TAVI (42-48).
Bioprosthesis type and size
Currently, two THV systems are available for implantation in Europe. The Edwards SAPIEN XT THV is a balloonexpandable valve that consists of a radiopaque cobaltchromium frame, trileaflet bovine pericardial leaflets, and a polyethylene terephthalate fabric skirt. The Edwards SAPIEN XT THV is currently available in 4 sizes (20, 23, 26, and 29 mm) and can be implanted in native annuli with diameters of 16 to 27 mm. The Medtronic CoreValve bioprosthesis is a self-expandable valve manufactured from a radiopaque nitinol support frame, trileaflet porcine pericardial leaflets, and porcine pericardium fabric skirt. The CoreValve is available in 4 sizes (23, 26, 29, and 31 mm) and can be implanted in native annuli with diameters ranging from 17 to 29 mm.
Comparisons between the two available bioprosthesis types are few (58,59). To date, appreciable differences between the systems include a higher incidence of new pacemaker requirement with the CoreValve device (53). Approximately 15-47% (60-62) and 4-21% (63,64) of patients require a new permanent pacemaker after CoreValve and Edwards SAPIEN implantation, respectively. Importantly, new pacemaker implantat ion does not appear to be associated with long-term mortality (65). Therefore, the decision to implant a particular bioprosthesis depends largely on the availability of the devices, the experience of the operator with each device, and pre-procedural anatomical screening.
Operator exper ience is a n impor tant factor in determining TAVI outcomes (66-68). Therefore, the majority of individual operators tend to implant a single bioprosthesis type while many high volume TAVI centres implant both valves. In these centres, bioprosthesis choice is dependant on the preprocedural assessment of the peripheral vasculature and the aortic annulus. Manufacture sizing guidelines, based on echocardiographic annulus measurement, are available (69). For the Edwards Sapien XT valve, the 20 mm valve is designed for small annuli between 16-19 mm, the 23 mm valve is designed for 18-21 mm annuli, the 26 mm valve for 22-25 mm annuli, and the larger 29 mm valve for 25-27 mm annuli. For the Medtronic CoreValve 23, 26, 29, and 31 mm bioprosthesis sizes are designed for annuli between 17-20, 20-23, 24-27, and 26-29 mm respectively.
Personal perspective on transcatheter aortic valve sizing
Appropriate oversizing of transcatheter aortic valves relative to the aortic annulus is needed for (I) anchoring to prevent migration; (II) sealing to prevent paravalvular aortic regurgitation; and (III) proper valve functioning to prevent pat ient-prosthesis mismatch. Current echocardiographic sizing guidelines for the Medtronic CoreValve and Edwards SAPIEN XT would suggest an oversizing percentage between 7-30% and 4-27%, respect ively (Table 2). Because self-expanding and balloon-expandable valves interfere differently with the aortic annulus, we should not expect similar oversizing principles. In our practice, MDCT dictates selection of the transcatheter aortic valve size. We compare the ratio of the aortic annulus perimeter obtained by MDCT to the perimeter of the transcatheter aortic valve (i.e. perimeter of the transcatheter aortic valve minus the perimeter of the aortic annulus then divided by the perimeter of the aortic annulus multiplied by 100). For self-expanding and balloon-expanding prostheses we aim for an oversizing percentage of 8-20% and 5-15%, respectively. Knowing that echocardiography underestimates the aortic annulus measurements, the actual oversizing obtained by echocardiography is less than expected. We believe that MDCT sizing allows a better approximation between the expected and actual oversizing than echocardiography.
| Table 2 Transcatheter aortic valve echocardiographic sizing and oversizing principles | ||||
| Valve size | Aortic valve annulus criteria | Absolute oversizing | Relative oversizing | |
| Medtronic coreValve | 26 mm |
20-23 mm |
3-6 mm |
13-30% |
| Edwards SAPIEN | 23 mm |
18-22 mm |
1-5 mm |
4-27% |
Finally, the choice of bioprosthesis can be influenced by the iliofemoral anatomy. The minimal femoral dimensions for the available TAVI systems are based on the French (Fr) size of the access sheaths and catheters. According to manufacture guidelines, the 18 Fr CoreValve and 22/24 Fr Edwards SAPIEN delivery sheaths require 6 mm, 7 and 8 mm diameter femoral arteries respectively. The newer Edwards SAPIEN XT system requires 6 mm and 6.5 mm femoral artery diameters for the 18 Fr and 19 Fr systems respectively.
Future perspectives
It is likely that, similar to the evolution of drug-eluting stents, an initial conservative approach during the TAVI regulatory approval process will be followed by off-label case selection and treatment of lower risk patients. The move towards treating lower risk patients has already emerged in Europe (70,71). Future trials such as SURTAVI and Partner 2 will explore TAVI in patients at intermediate operative risk.
Although risk scores continue to play an important role in guiding patient selection for TAVI, they are poorly suited to this task. The development of alternative risk models designed specifically for high-risk TAVI recipients (72), and perhaps incorporating a measure of frailty, are required. Furthermore, the integration of MDCT in peripheral screening and most importantly, in annulus measurement for bioprosthesis sizing, is likely to improve outcomes in TAVI recipients.
Conclusions
Patient selection for TAVI is of considerable importance in optimizing procedural and long-term outcomes. The multi-disciplinary heart team approach, and the use of multimodal imaging is strongly advocated. Annulus sizing using MDCT is emerging as the modality of choice for assessment of annulus size and bioprosthesis sizing. Surgical risk scores must be refined to represent the unique challenge posed by high-risk TAVI populations, and incorporate a measure of frailty.
Acknowledgements
Disclosure: Dr Piazza is a consultant for Medtronic.
References
- Piazza N, de Jaegere P, Manoharan G, et al. Patient Selection for the CoreValve ReValving System. In: Serruys P, Piazza N, Cribier A, et al, editors. Transcatheter aortic valve implantation: Tips and tricks to avoid failure. New York: Informa Healthcare, 2011:82.
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/ SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement: Developed in collaboration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Ann Thorac Surg 2012;93:1340-95.
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68.
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:e523-661.
- Grayburn PA. Assessment of low-gradient aortic stenosis with dobutamine. Circulation 2006;113:604-6.
- Lancellotti P, Lebois F, Simon M, et al. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005;112:I377-82.
- Roques F, Nashef SA, Michel P. Risk factors for early mortality after valve surgery in Europe in the 1990s: lessons from the EuroSCORE pilot program. J Heart Valve Dis 2001;10:572-7, discussion 577-8.
- Shroyer AL, Coombs LP, Peterson ED et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg 2003;75:1856-64; discussion 1864-5.
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62-9.
- Dewey TM, Brown D, Ryan WH, et al. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008;135:180-7.
- O’Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg 2009;88:S23-42.
- Osswald BR, Gegouskov V, Badowski-Zyla D, et al. Overestimation of aortic valve replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur Heart J 2009;30:74-80.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Lefèvre T, Kappetein AP, Wolner E, et al. One year followup of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J 2011;32:148-57.
- Buellesfeld L, Gerckens U, Schuler G, et al. 2-year followup of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol 2011;57:1650-7.
- Delgado V, Ewe SH, Ng AC, et al. Multimodality imaging in transcatheter aortic valve implantation: key steps to assess procedural feasibility. EuroIntervention 2010;6:643-52.
- Eltchaninoff H, Kerkeni M, Zajarias A, et al. Aorto-iliac angiography as a screening tool in selecting patients for transfemoral aortic valve implantation with the Edwards SAPIEN bioprosthesis. EuroIntervention 2009;5:438-42.
- Hayashida K, Lefevre T, Chevalier B, et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv 2011;4:851-8.
- Nietlispach F, Leipsic J, Al-Bugami S, et al. CT of the iliofemoral arteries using direct aortic contrast injection: proof of feasibility in patients screened towards percutaneous aortic valve replacement. Swiss Med Wkly 2009;139:458-62.
- Wuest W, Anders K, Schuhbaeck A, et al. Dual source multidetector CT-angiography before Transcatheter Aortic Valve Implantation (TAVI) using a high-pitch spiral acquisition mode. Eur Radiol 2012;22:51-8.
- Piazza N, de Jaegere P, Schultz C, et al. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Intervent 2008;1:74-81.
- Kodali SK, Williams MR, Smith CR, et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med 2012;366:1686-95.
- Messika-Zeitoun D, Serfaty JM, Brochet E, et al. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol 2010;55:186-94.
- Tzikas A, Schultz CJ, Piazza N, et al. Assessment of the aortic annulus by multislice computed tomography, contrast aortography, and trans-thoracic echocardiography in patients referred for transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2011;77:868-75.
- Husser O, Rauch S, Endemann DH, et al. Impact of three-dimensional transesophageal echocardiography on prosthesis sizing for transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2012. [Epub ahead of print].
- Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J 2012;32:2189-214.
- Schultz CJ, Moelker A, Piazza N, et al. Three dimensional evaluation of the aortic annulus using multislice computer tomography: are manufacturer’s guidelines for sizing for percutaneous aortic valve replacement helpful? Eur Heart J 2010;31:849-56.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56.
- Makkar RR. Two-Year Outcomes of Transcatheter Aortic Valve Replacement (TAVR) in “Inoperable” Patients With Severe Aortic Stenosis: The PARTNER Trial. Transcatheter Cardiovascular Therapeutics (TCT). San Francisco, California, 2011.
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7.
- Saia F, Marrozzini C, Moretti C, et al. The role of percutaneous balloon aortic valvuloplasty as a bridge for transcatheter aortic valve implantation. EuroIntervention 2011;7:723-9.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009;2:828-33.
- Bauernschmitt R, Schreiber C, Bleiziffer S, et al. Transcatheter aortic valve implantation through the ascending aorta: an alternative option for no-access patients. Heart Surg Forum 2009;12:E63-4.
- Taramasso M, Giacomini A, Maisano F. Transcatheter aortic valve implantation through the left subclavian artery with a patent LIMA graft. Catheter Cardiovasc Interv 2010;76:153-5.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010;3:359-66.
- Sharp AS, Michev I, Colombo A. First trans-axillary implantation of Edwards Sapien valve to treat an incompetent aortic bioprosthesis. Catheter Cardiovasc Interv 2010;75:507-10.
- Bruschi G, Fratto P, De Marco F, et al. The transsubclavian retrograde approach for transcatheter aortic valve replacement: single-center experience. J Thorac cardiovasc surg 2010;140:911-5, 915,e1-2.
- De Marco F, Bruschi G, Klugmann S. Transcatheter aortic valve implantation by left subclavian access in the presence of a patent LIMA to LAD graft. Catheter Cardiovasc Interv 2011;77:430-4.
- Modine T, Obadia JF, Choukroun E, et al. Transcutaneous aortic valve implantation using the axillary/subclavian access: feasibility and early clinical outcomes. J Thorac cardiovasc surg 2011;141:487-91, 491, e1.
- Vavuranakis M, Vrachatis DA, Filis K, et al. Trans-catheter aortic-valve implantation by the subclavian approach complicated with vessel dissection and transient left-arm paralysis. Eur J Cardiothorac Surg 2011;39:127-9.
- Guarracino F, Covello RD, Landoni G, et al. Anesthetic management of transcatheter aortic valve implantation with transaxillary approach. J Cardiothorac Vasc Anesth 2011;25:437-43.
- Bapat V, Thomas M, Hancock J, et al. First successful trans-catheter aortic valve implantation through ascending aorta using Edwards SAPIEN THV system. Eur J Cardiothorac Surg 2010;38:811-3.
- Bruschi G, De Marco F, Fratto P, et al. Direct aortic access through right minithoracotomy for implantation of selfexpanding aortic bioprosthetic valves. J Thorac Cardiovasc Surg 2010;140:715-7.
- Latsios G, Gerckens U, Grube E. Transaortic transcatheter aortic valve implantation: a novel approach for the truly “no-access option” patients. Catheter Cardiovasc Interv 2010;75:1129-36.
- Bruschi G, De Marco F, Fratto P, et al. Alternative approaches for trans-catheter self-expanding aortic bioprosthetic valves implantation: single-center experience. Eur J Cardiothorac Surg 2011;39:e151-8.
- Etienne PY, Papadatos S, El Khoury E, et al. Transaortic transcatheter aortic valve implantation with the Edwards SAPIEN valve: feasibility, technical considerations, and clinical advantages. Ann Thorac Surg 2011;92:746-8.
- Etienne PY, Papadatos S, Pieters D, et al. Embol-x intraaortic filter and transaortic approach for improved cerebral protection in transcatheter aortic valve implantation. Ann Thorac Surg 2011;92:e95-6.
- Cockburn J, Trivedi U, Hildick-Smith D. Transaortic transcatheter aortic valve implantation within a previous bioprosthetic aortic valve replacement. Catheter Cardiovasc Interv 2011;78:479-84.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011;124:S124-9.
- D’Onofrio A, Rubino P, Fusari M, et al. Clinical and hemodynamic outcomes of “all-comers” undergoing transapical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation (I-TA). J Thorac Cardiovasc Surg 2011;142:768-75.
- Falk V, Walther T, Schwammenthal E, et al. Transapical aortic valve implantation with a self-expanding anatomically oriented valve. Eur Heart J 2011;32:878-87.
- Kempfert J, Rastan AJ, Mohr FW, et al. A new selfexpanding transcatheter aortic valve for transapical implantation - first in man implantation of the JenaValve. Eur J Cardiothorac Surg 2011;40:761-3.
- Moat NE, Ludman P, de Belder MA, et al. Long-Term Outcomes After Transcatheter Aortic Valve Implantation in High-Risk Patients With Severe Aortic Stenosis: The U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8.
- Erdoes G, Basciani R, Huber C, et al. Transcranial Doppler-detected cerebral embolic load during transcatheter aortic valve implantation. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery 2012;41:778-84.
- Himbert D, Descoutures F, Al-Attar N, et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol 2009;54:303-11.
- Ewe SH, Delgado V, Ng AC, et al. Outcomes after transcatheter aortic valve implantation: transfemoral versus transapical approach. Ann Thorac Surg 2011;92:1244-51.
- Dewey TM, Thourani V, Bavaria JE, et al. Transapical aortic-valve replacement for critical aortic stenosis: Results from the nonrandomized continued-access cohort of the PARTNER trial. Society of Thoracic Surgeons Annual Meeting. Ft Lauderdale, FL., 2012.
- Bosmans JM, Kefer J, De Bruyne B, et al. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg 2011;12:762-7.
- Jilaihawi H, Chakravarty T, Weiss RE, et al. Meta-analysis of complications in aortic valve replacement: Comparison of Medtronic-Corevalve, Edwards-Sapien and surgical aortic valve replacement in 8,536 patients. Catheter Cardiovasc inter 2012;80:128-38.
- Bleiziffer S, Ruge H, Horer J, et al. Predictors for newonset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:524-30.
- Jilaihawi H, Chin D, Vasa-Nicotera M, et al. Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis. Am Heart J 2009;157:860-6.
- Piazza N, Onuma Y, Jesserun E, et al. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv 2008;1:310-6.
- Gutiérrez M, Rodés-Cabau J, Bagur R, et al. Electrocardiographic changes and clinical outcomes after transapical aortic valve implantation. Am Heart J 2009;158:302-8.
- Sinhal A, Altwegg A, Pasupati S, et al. Atrioventricular block after transcatheter balloon expandable aortic valve implantation. JACC Cardiovasc Interv 2008;1:305-9.
- D’Ancona G, Pasic M, Unbehaun A, et al. Permanent pacemaker implantation after transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg 2011;13:373-6.
- Nuis RJ, van Mieghem NM, van der Boon RM, et al. Effect of experience on results of transcatheter aortic valve implantation using a Medtronic CoreValve System. Am J Cardiol 2011;107:1824-9.
- Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valverelated outcomes. Circulation 2009;119:3009-16.
- Grube E, Buellesfeld L, Mueller R, et al. Progress and Current Status of Percutaneous Aortic Valve Replacement: Results of Three Device Generations of the CoreValve Revalving System. Circ Cardiovasc Intervent 2008;1:167-75.
- Jilaihawi H, Bonan R, Asgar A, et al. Anatomic suitability for present and next generation transcatheter aortic valve prostheses: evidence for a complementary multidevice approach to treatment. JACC Cardiovasc Interv 2010;3:859-66.
- Piazza N, Otten A, Schultz C, et al. Adherence to patient selection criteria using the third generation 18F CoreValve ReValving System. Heart 2010;96:19-26.
- Lange R, Bleiziffer S, Mazzitelli D, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol 2012;59:280-7.
- Van Mieghem NM, Head SJ, van der Boon RM, et al. The SURTAVI model: proposal for a pragmatic risk stratification for patients with severe aortic stenosis. EuroIntervention 2012. [Epub ahead of print].





