Video assisted thoracoscopic excision of mediastinal ectopic parathyroid adenomas: a UK regional experience
Introduction
Mediastinal ectopic parathyroid adenomas (MEPAs) are rare tumors, constituting 1-2% of all parathyroid adenomas (1-3). Thorough understanding of the anatomy and embryology of the parathyroid glands is required by endocrine and thoracic surgeons to maximise chances of cure from hyperparathyroidism (4-6). It is also of paramount importance that clinicians should be aware of the advancements in preoperative parathyroid localization, intraoperative parathyroid hormone (PTH) monitoring and the different surgical accesses for MEPAs, including ‘focused’ parathyroidectomy, bilateral surgical neck exploration, video-assisted neck exploration, radioguided parathyroidectomy, feeding vessel embolization, bronchoscopic ablation, open thoracotomy, median sternotomy and video-assisted thoracoscopic surgery (VATS). The dictum that ‘the best treatment of hyperparathyroidism is to locate a qualified surgeon’ still holds true (7). Surgical exploration of the neck by endocrine surgeons is very popular, and many patients are referred to thoracic or specialised endocrine surgeons only after one or bilateral failed neck explorations. The traditional approach to MEPAs has been a trans-sternal approach or lateral thoracotomy (1-3). VATS is now an established minimally invasive technique for the resection of intra-thoracic lesions (8,9). We report our regional experience with seven patients, six of whom underwent successful VATS excision of MEPAs.
Methods
Between January 2004 and December 2009, seven patients presented with mediastinal single gland ectopic hyperparathyroidism. Six patients had primary hyperparathyroidism and one had tertiary hyperparathyroidism. All patients had computed tomography (CT) scans of the chest. In addition, four patients had a Tc99m-Sestamibi scan and single photon emission computed tomography (SPECT) scans.
Excision was performed via VATS with the patient in the supine position, arms by the side, except for two patients with right paratracheal adenoma who were approached in the full lateral position. Single lung ventilation was established via a double lumen tube, while lung collapse was assisted by carbon dioxide (CO2) insufflation in two patients. Intrapleural pressure was maintained at 10 mmHg at low flow, with the anesthetist continuously monitoring hemodynamics. Three ports were fashioned for access: 10 mm for the camera, 5 mm for instruments and 10 mm utility port.
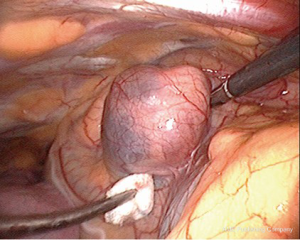
Intravenous methylene blue (MB) at a dose of 0.5 mg/kg body weight in a 500 mL bag of 5% dextrose/saline was started intravenously at induction of anesthesia and finished just before exploration (Figures 1,2) (10). MEPAs preferentially took up the dye and were made easily identifiable at operation. In 5/7 cases, the chest was drained overnight with a single intercostal tube. One patient had no drain and the converted case had two drains. Morphine was used to maintain postoperative analgesia through intravenous patient controlled analgesia (PCA), and local port blocks using bupivacaine 0.5%. The endocrine medical team were closely involved with the postoperative care of patients, with corrected calcium and PTH serum level monitored immediately after the operation. The serum calcium level was measured daily until the patient was discharged (1-7 days), and the family doctor was instructed to repeat measurements of the calcium level on discharge.

Results
There were five women and two men with a mean age of 53 years (range, 27-72 years). Table 1 summarises their demographic characteristics. Three patients had undergone prior cervical explorations. There was one conversion to open thoracotomy due to brisk bleeding caused by excessive traction on a slung azygos vein. All patients were extubated shortly after surgery and none required escalated monitoring in the intensive care or high dependency unit. Postoperative plasma calcium returned to normal in all six patients with preoperative hypercalcemia. The PTH level returned to normal in 6/7 patients but remained consistently high in the seventh. This patient had unsuspected and extensive tuberculous lymphadenopathy which concentrated the MB throughout the mediastinal lymph nodal chain. An adenoma could not be found either by VATS or on open conversion. Histopathology confirmed a successfully resected parathyroid adenoma in the other six cases.
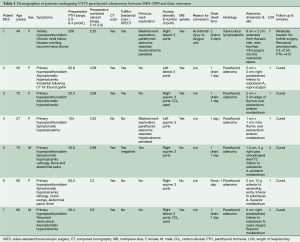
Full table
The median hospital stay was 2 days with a range of 1-7 days. Apart from the technical azygos bleeding, there were no procedure related complications such as hemothorax, pneumonia, wound infection, arrhythmia, hypocalcaemic tetany, recurrent laryngeal nerve palsy, or MB toxicity.
Table 2 shows the cohort of patients with parathyroidism who underwent surgical operations during the same time frame [2005-2009] at Southampton General Hospital, a tertiary referral centre. A total of 141 patients were operated upon for hyperparathyroidism in 6 years. Of these, 132 had either unilateral or bilateral neck exploration. Two had median sternotomy, and seven underwent VATS (this series). Histology showed that the adenoma was missed in two cases, and was malignant in only one case. Interestingly, this malignant case had recurrence of hypercalcemia after bilateral successful neck and chest exploration.
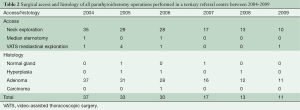
Full table
Discussion
Parathyroid glands were first described in humans by a medical student from Uppsala-Sweden called Ivar Sandström in 1879 (7). They are usually four in number, bean shaped, yellow-tan in colour, 5 mm across and less than 35 g in weight. They consist of three cell types: the chief cell, the oxyphil cell, and the clear cell. The chief and oxyphil cells produce PTH and the function of the clear cells is unknown. They develop roughly around the second month of gestation under the base of the skull, around the developing thymus and thyroid glands. They migrate down the neck and attain a position related to the inferior poles of thyroid gland in the neck. They are usually found within the capsule of the thyroid, surrounded by adipose tissue, and can be difficult to identify. If they fail to descend, they are found high in the neck under the mandible (undescended). On the other hand, if they descend too far, they are found within the mediastinum (ectopic). The superior parathyroid glands undergo very little migration, and their location is rather constant whereas the inferior ones migrate with the thymic primordium and can be ectopic. Supernumerary parathyroid glands can exist within thymic tissue. The commonest ectopic sites are on or near the thymus, superior and anterior mediastinum and aorto-pulmonary window (APW).
Parathyroid glands secrete PTH, which regulates calcium and phosphate levels in blood. Hyperparathyroidism can be primary due to diseased parathyroid glands, or secondary due to disease elsewhere, usually kidneys. Primary hyperparathyroidism can result from benign parathyroid adenomas, hyperplasia of one or more parathyroid glands or rarely cancerous gland. Eighty percent of patients are asymptomatic or have minor symptoms related to hypercalcemia, such as fatigue, loss of appetite, nausea, constipation, excessive thirst, mild depression and loss of concentration. With high calcium levels, patients may develop bilateral recurrent renal stones, bone resorption and fractures, and rheumatological complications. Secondary hyperparathyroidism refers to the excessive secretion of PTH by the parathyroid glands in response to hypercalcaemia and is associated with hyperplasia of the glands. This disorder is especially seen in patients with chronic renal failure. Tertiary hyperparathyroidism, is a state of excessive secretion of PTH resulting from a long period of secondary hyperparathyroidism culminating in autonomous (unregulated) parathyroid function.
In reported series of primary hyperparathyroidism, the incidence of MEPAs is about 1-2% (1-3). The Mayo clinic reported 33 cases of mediastinal parathyroidectomy between March 1980 and September 2010, a 30 years span (8). The two hospitals involved in this report serve a population of 3.3 million in the South West of the United Kingdom and report seven cases in six years (Table 2). In the same reporting period, there have been 132 cases of cervical exploration for hyperthyroidism and two sternotomies for MEPAs.
There is no consensus on specific tests that should be routinely performed in hyperparathyroidism, however. MEPAs can be detected by a range of localising techniques including imaging and selective venous sampling for high levels of PTH. Ultrasonography, CT scan, magnetic resonance imaging (MRI), positron emission tomography (PET), and recently Technetium (Tc99m-Sestamibi) scan are becoming standard. SPECT is combined with sestamibi scintigraphy to provide three-dimensional imaging. The reported sensitivity of Tc99m-Sestamibi is up to 90% (11-15). Three-dimensional Multi Planar Reconstruction of images could also be reconstructed from CT scans.
Until recently, bilateral neck exploration was the gold standard operation for primary hyperparathyroidism, without localisation imaging. The Scholz-Purnell report from the Mayo Clinic in 1971 and the Kaiser report by Rubinoff in 1983 concluded that: “our recommendation for patients whose clinical and laboratory studies support the diagnosis of primary hyperparathyroidism has been and continues to be surgical exploration by an experienced parathyroid surgeon.” There is general agreement that an experienced parathyroid surgeon should perform a minimum of nine to ten explorations annually (16,17). Bilateral neck exploration aims at identifying all four parathyroid glands and removing all abnormal parathyroid tissue. Despite a high cure rate approaching 70%, neck exploration is related to failure, morbidities and difficult second surgeries, not to mention the large unsightly incision. Edis et al. suggested that the main reason for failed exploration is “failure on the part of the surgeon to appreciate the nuances and variations of normal parathyroid anatomy” (18). This trend is retracting, as precise preoperative localisation studies and intraoperative PTH monitoring coupled with the advent of keyhole surgery (VATS) have managed to improve cure rates to 95-98%. Such a novel and precise approach for a single gland hyperparathyroidism renders exploration of the normal glands unnecessary.
Anatomical mediastinal location of the tumor has dictated the surgical approach. Traditionally before the introduction of VATS, mediastinal MEPAs were resected by thoracotomy or median sternotomy (1-3,19). VATS offers significant advantages over both (8,9). It allows better visualisation of the tumor, a shorter operative time, shorter tube dwell time and shorter hospital stay. Informed patients prefer the superior cosmetic result compared to sternotomy and thoracotomy scars. We encountered the ectopic adenomas within the thymus tissue or closely related to it (Figure 2), as could be expected from the embryological close relationship of the two organs (10). On the right, it was also seen in a paratracheal position within the superior triangle (bound by the superior vena cava, azygos vein and vagus nerve). The triangle is occupied by the fibro-fatty-nodal block that contains R2-4 nodes. Discoloration by MB differentiated it from lymph nodes in the area ((Figure 2) (10). Figure 2 shows the adenoma could be directly related to the pericardium (10). This absence of thymic tissue raises the possibility that the ectopic parathyroids of the middle mediastinum arose from parathyroid IV. Gilmour noted that parathyroid IV is in contact with the pericardium in the 3 mm embryo and it is possible that some parathyroid precursor cells may retain this relationship and develop in the middle mediastinum (5).
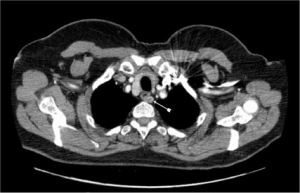
All adenomas in this series would have been outside the remit of cervical access. However; there is no clear ‘cut off’ landmark favouring VATS over cervical access. Liaison between an expert endocrine surgeon and a thoracic surgeon expert in VATS mediastinal surgery is recommended. Roughly, MEPAs higher than the right innominate artery or the origin of the left subclavian artery are likely to be accessible by a cervical approach (Figure 3). However, it is to be emphasized that there is little evidence to support this generalisation. Wheeler et al. (Cardiff-1988) described the challenges of the middle mediastinal parathyroid adenomas (20). The three cases described in their series would have been the domain of VATS nowadays without the need for open sternotomy (Figure 4).

Other unusual sites in the chest have been reported. Ali et al. and Arnault et al. reported MEPAs in the APW, using CT and superselective sampling of inferior thyroid venous blood (21,22). They successfully treated these adenomas by embolization of the feeding artery, which is usually a bronchial artery. MEPAs can rarely be endobronchial, masquerading as asthma and treated by laser ablation or bronchoplastic procedure (23).
MB injected at induction of anesthesia is our preferred method of intraoperative localisation of the ectopic adenoma. Dudley et al. used it as early as 1971 to identify the parathyroids intraoperatively (24). It has proven to be a simple and safe method of intraoperative visual localisation of MEPAs. MB is a cationic thiazine dye with a variety of uses including treatment of drug-induced methaemoglobinaemia, septic shock, treatment and prevention of ifosfamide—induced encephalopathy (25,26). It is primarily excreted in the urine after reduction by glucose-6 phosphate dehydrogenase (G6PD) (27). MB effectively functions as a potent competitive inhibitor of monoamine oxidase A (MAOI) and the combination of MB with a serotonergic drug predisposes to serotonin toxicity, therefore such medication should be avoided if MB were to be used. We used infusions of up to 200 mg in 500 mL of 5% dextrose/saline started on induction of anesthesia and finished before surgical exploration. Tummers et al. advocate a dose of 0.5 mg/kg as safe (28). We have not encountered any of the possible complications such as confusional state, hypoxia or allergic reactions (29). Liker has reported prolonged time of recovery from anesthesia after intravenous MB infusion in patients undergoing parathyroidectomy (30). Unfortunately, the specificity of MB in our experience was not 100%, as a tuberculous node confounded the results in one patient. However, in the majority of cases these adenomas are too obvious to miss, and we speculate that MB would be a useful adjunct in locating small adenomas found within lymph nodal field such as the APW. This technique is helpful visual marker but does not replace intraoperative PTH (ioPTH) monitoring when dissecting around nodes in the APW.
IOPTH monitoring consists of analysis of preoperative, pre-excision, and post-excision hormonal assay of PTH at 5 and 10 min following surgical removal of suspected adenoma. Blood samples are obtained from a peripheral vein or the internal jugular vein. The positive predictive criteria for prediction of cure are a drop in ioPTH by >50% of pre-excision values. Lack of adequate drop after targeted resection should trigger continued exploration (6,31). The turn round time in theatre of this hormonal assay is usually about 20 min. There is still some controversy about the criteria of ioPTH assay that can predict cure.
Case number 1 demonstrates that failed exploration could be followed by successful biochemical control of mild symptoms. This is in keeping with The National Institute for Health and Clinical Excellence (NICE) guidelines (32). The presumed MEPA proved to be a tuberculous lymph node and the patient received anti-tuberculous medication. She never proceeded to a second exploration despite high levels of PTH. She is alive and well 10 years after the operation.
MEPAs are rare tumors and as such should be dealt within a specialist tertiary referral centre. NICE has published an interventional procedure consultation document on thoracoscopic excision of mediastinal parathyroid tumors in 2007 (32). It has concluded that the evidence on VATS safety is very limited in quantity, and in view of potential complications of the procedure it should only be used with special arrangements for clinical governance, consent, audit and research. Better patient outcomes have been reported when complex surgical procedures are performed at high-volume hospitals and centres of excellence. This phenomenon was first noted in 1979, when Luft et al. reported that the mortality rate for certain surgeries was inversely proportional to the number of procedures performed (33). Because of a paucity of cases, experience should be concentrated within tertiary referral hospitals. The choice between access with the patient supine or on the lateral decubitus position is subtle and reflects the need for such experience. Intraoperative experience with CO2 insufflation and monitoring of hemodynamics is another example. Which cases would require MB and ioPTH monitoring for completion of resection is not readily appreciated by the occasional operator. Detailed knowledge of the anatomy of APW and relations of the left recurrent laryngeal nerve remains the domain of thoracic surgeons competent in mediastinal VATS surgery.
We conclude that MEPAs can be safely resected by VATS with minimal surgical morbidity, short procedure and short hospital stay. Chest drainage is either not necessary or could be as short as few hours postoperatively. CO2 insufflation and intraoperative use of MB are safe and help to accurately localise the ectopic parathyroid tissue. Patients with mediastinal adenoma differ substantially from cervical cases and require a specific strategy. VATS should be considered as the first line approach for resection of these ectopic tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Conn JM, Goncalves MA, Mansour KA, et al. The mediastinal parathyroid. Am Surg 1991;57:62-6. [PubMed]
- Russell CF, Edis AJ, Scholz DA, et al. Mediastinal parathyroid tumors: experience with 38 tumors requiring mediastinotomy for removal. Ann Surg 1981;193:805-9. [PubMed]
- Downey NJ, McGuigan JA, Dolan SJ, et al. Median sternotomy for parathyroid adenoma. Ir J Med Sci 1999;168:13-6. [PubMed]
- Weller G. Development of the thyroid, parathyroid and thymus glands in man. Contrib Embryol 1933;24:93-142.
- Gilmour JR. The embryology of the parathyroid glands, the thymus and certain associated rudiments. The Journal of Pathology and Bacteriology 2005;45:507-22.
- Phitayakorn R, McHenry CR. Parathyroidectomy: overview of the anatomic basis and surgical strategies for parathyroid operations. Clin Rev Bone Miner Metab 2007;5:89-102.
- Organ CH Jr. The history of parathyroid surgery, 1850-1996: the Excelsior Surgical Society 1998 Edward D Churchill Lecture. J Am Coll Surg 2000;191:284-99. [PubMed]
- Said SM, Cassivi SD, Allen MS, et al. Minimally invasive resection for mediastinal ectopic parathyroid glands. Ann Thorac Surg 2013;96:1229-33. [PubMed]
- Sukumar MS, Komanapalli CB, Cohen JI. Minimally invasive management of the mediastinal parathyroid adenoma. Laryngoscope 2006;116:482-7. [PubMed]
- Amer K, Khan AZ, Rew D, et al. Video assisted thoracoscopic excision of mediastinal ectopic parathyroid adenomas: a UK regional experience. Asvide 2015;2:144. Available online: http://www.asvide.com/articles/721
- Krausz Y, Bettman L, Guralnik L, et al. Technetium-99m-MIBI SPECT/CT in primary hyperparathyroidism. World J Surg 2006;30:76-83. [PubMed]
- Rubello D, Pagetta C, Piotto A, et al. Efficacy of sequential double tracer subtraction and SPECT parathyroid imaging in the precise localization of a low mediastinal parathyroid adenoma successfully removed surgically. Clin Nucl Med 2004;29:662-3. [PubMed]
- Smith JR, Oates ME. Radionuclide imaging of the parathyroid glands: patterns, pearls, and pitfalls. Radiographics 2004;24:1101-15. [PubMed]
- Amar L, Guignat L, Tissier F, et al. Video-assisted thoracoscopic surgery as a first-line treatment for mediastinal parathyroid adenomas: strategic value of imaging. Eur J Endocrinol 2004;150:141-7. [PubMed]
- Rubello D, Pelizzo MR, Boni G, et al. Radioguided surgery of primary hyperparathyroidism using the low-dose 99mTc-sestamibi protocol: multi-institutional experience from the Italian Study Group on Radioguided Surgery and Immunoscintigraphy (GISCRIS). J Nucl Med 2005;46:220-6. [PubMed]
- Purnell DC, Smith LH, Scholz DA, et al. Primary hyperparathyroidism: a prospective clinical study. Am J Med 1971;50:670-8. [PubMed]
- Rubinoff H, McCarthy N, Hiatt RA. Hypercalcemia: long-term follow-up with matched controls. J Chronic Dis 1983;36:859-68. [PubMed]
- Edis AJ, Sheedy PF, Beahrs OH, et al. Results of reoperation for hyperparathyroidism, with evaluation of preoperative localization studies. Surgery 1978;84:384-93. [PubMed]
- Obara T, Fujimoto Y, Tanaka R, et al. Mid-mediastinal parathyroid lesions: preoperative localization and surgical approach in two cases. Jpn J Surg 1990;20:481-6. [PubMed]
- Curley IR, Wheeler MH, Thompson NW, et al. The challenge of the middle mediastinal parathyroid. World J Surg 1988;12:818-24. [PubMed]
- Ali M, Kumpe DA. Embolization of bronchial artery-supplied ectopic parathyroid adenomas located in the aortopulmonary window. J Vasc Interv Radiol 2014;25:138-43. [PubMed]
- Arnault V, Beaulieu A, Lifante JC, et al. Multicenter study of 19 aortopulmonary window parathyroid tumors: the challenge of embryologic origin. World J Surg 2010;34:2211-6. [PubMed]
- Özgül MA, Seyhan EC, Özgül G, et al. Endotracheal ectopic parathyroid adenoma mimicking asthma. Respir Med Case Rep 2014;13:28-31. [PubMed]
- Dudley NE. Methylene blue for rapid identification of the parathyroids. Br Med J 1971;3:680-1. [PubMed]
- Rowley M, Riutort K, Shapiro D, et al. Methylene blue-associated serotonin syndrome: a 'green' encephalopathy after parathyroidectomy. Neurocrit Care 2009;11:88-93. [PubMed]
- Vutskits L, Briner A, Klauser P, et al. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008;108:684-92. [PubMed]
- Clifton J 2nd, Leikin JB. Methylene blue. Am J Ther 2003;10:289-91. [PubMed]
- Tummers QR, Schepers A, Hamming JF, et al. Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose Methylene Blue. Surgery 2015;158:1323-30. [PubMed]
- Khan MA, North AP, Chadwick DR. Prolonged postoperative altered mental status after methylene blue infusion during parathyroidectomy: a case report and review of the literature. Ann R Coll Surg Engl 2007;89:W9-11. [PubMed]
- Licker M, Diaper J, Robert J, et al. Effects of methylene blue on propofol requirement during anaesthesia induction and surgery. Anaesthesia 2008;63:352-7. [PubMed]
- Sagan D, Goździuk K. Surgical treatment of mediastinal parathyroid adenoma: rationale for intraoperative parathyroid hormone monitoring. Ann Thorac Surg 2010;89:1750-5. [PubMed]
- NICE interventional procedure guidance. Thoracoscopic excision of mediastinal parathyroid tumours. 2007. Available online: http://www.nice.org.uk/guidance/ipg247
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364-9. [PubMed]





