Video-assisted thoracoscopic microthymectomy
Introduction
There is a wide range of techniques that may be employed in order to achieve a minimally invasive thymectomy. In our journey through the range of techniques available, we had at the core of our ethos the principles laid out by the International Thymic Malignancy Interest Group (ITMIG) in 2011, which requires an en bloc thymectomy for all thymomas, including perithymic fat and cervical poles. Furthermore, patients with myasthenia gravis are required to undergo an extended thymectomy must be performed, including the mediastinal pleura, pericardiophrenic fatty tissue and dissection of the aorto-pulmonary window (1). The Myasthenia Gravis Foundation of America have also graded the completeness of thymectomy and provided a classification system, ranging from a T1. We are mindful of the need to achieve at least a T2b video-assisted thoracoscopic (VATS) extended thymectomy for myasthenic patients (2). In addition, we have been particularly impressed by subxiphoid approaches to thoracic surgery. Early descriptions in thoracic surgery were described by Mineo and colleagues (3,4) for metastasectomy, where two ports were placed in the intercostal spaces. The xiphisternum was then resected and an 8-cm vertical incision was made in the linea alba allow entry of the surgeons hand into the hemithorax in order to palpate the whole lung for metastases, as well as the contralateral hemithorax. More recently this approach has been replicated but with a single subxiphoid approach, avoiding all incisions between the intercostal spaces and using a Covidien single incision laparoscopic surgery (SILS) port and carbon dioxide (CO2) to perform bilateral metastasectomy (5). Cardiac surgery has also been performed using a subxiphoid-only approach by Mark Levinson, including multi vessel off pump coronary bypass and atrial septal defect repairs, using a 7-cm subxiphoid incision and a sternal elevator (6,7). Subxiphoid surgery has been extended to lobectomy of every lobe by Liu et al. from Taiwan using a sternal elevator rather than CO2 (8). They have also performed segmentectomies in this fashion. Resection of anterior mediastinal masses have been described with a subxiphoid incision (9) and thymectomy has also been performed using a single subxiphoid approach (10). This has been achieved using the SILS port and CO2, but also required a Kirschner wire in the upper sternum for sternal elevation.
Therefore, we aimed to reduce the magnitude of our own incisions both in lobectomy and thymectomy by using a subxiphoid utility port. However, subxiphoid-only surgery is technically very demanding as it is a considerable change compared to our usual multiport approach. In addition, we felt that this compromised safety and vision significantly in our hands. Subsequently, we have replicated our traditional VATS approaches but with a subxiphoid utility incision, which has allowed us to restrict ourselves to 5-mm ports rather than our usual 12-mm ports.
Operative techniques
Positioning
The patient is supine and the surgeon and assistant are by the patient’s side. If the patient has a thymoma that is more on the left side or near the left phrenic nerve, then we will start the operation on the left side (Figure 1A). However, for patients with non-thymomatous myasthenia gravis and in general, we will typically start the procedure in the right hemithorax (Figure 1B).
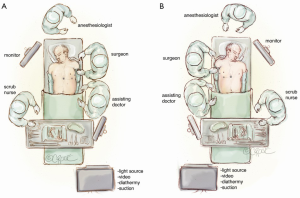
Port insertion for microthymectomy
The intercostal spaces range in size from 7-10 mm, and therefore large ports and instruments may easily cause damage to intercostal nerves, causing post-thoracostomy pain. Most large stapling devices require either a 12-mm or a 15-mm port for access, and thus in a standard VATS lobectomy 12 mm ports routinely used. Once a subxiphoid port is utilized, it may quickly be found that there is no necessity for large ports in the chest. In addition, high quality 5-mm cameras are now available and there is therefore no longer any need for 10-mm cameras in routine practice. Thus, our approach is as follows.
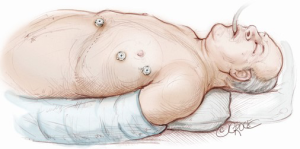
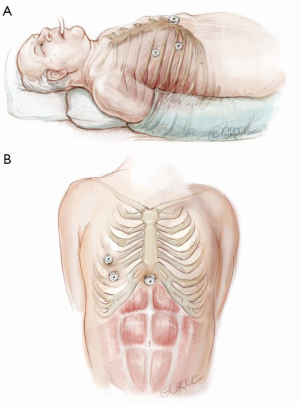
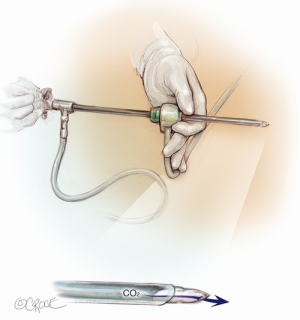
The computed tomography (CT) scan is assessed and this guides the placement of the two 5 mm intercostal ports. Figure 2 demonstrates the position of the left sided ports. However, in non thymomatous myasthenia gravis or in more right-sided thymomas, we prefer to approach from the right hemithorax due to the increased space on this side (Figure 3). The patient is positioned supine, often with a support under the left scapula in a left sided approach. A single lumen tube is used, as we use CO2 to create space in the anterior mediastinum bilaterally, so that single lung isolation is not required. The CO2 is set at 8 mmHg at a maximum flow rate of 8 litres per minute and reduced if there is hypotension, high airway pressures or hypercarbia. As a finger cannot be first inserted into the chest for a 5-mm port, we use a disposable plastic 5-mm port, which has a clear central trocar to enter the chest. (Figure 4). The CO2 is attached to the side arm and a 5-mm straight camera is inserted into the trocar. An incision is made over the skin of the 5th space in the anterior axillary line, and under screen vision and CO2 insufflation, the trocar is pushed into the chest once ventilation has been paused. The pleura and then the lung are seen on the screen and further advancement is paused as the lung is pushed away by the CO2. The port is then inserted safely into the chest. We have a camera cover that facilitates easy exchange with the straight and 30 degree cameras, and thus we now change to the 30 degree camera for the remainder of the procedure. After CO2 insufflation has pushed the diaphragm inferiorly, a second port is then placed in the mid clavicular line, usually in the 7th intercostal space. A 5-mm dissecting peanut is then used to push the mediastinal tissue away from the back of the inferior sternum in preparation for the subxiphoid incision. A 2-cm vertical incision is then made below the xiphisternum and down to the linea alba. The linea alba is divided by electrocautery for 2 cm and then a finger is inserted and pushed up vertically under the xiphisternum and body of the sternum. The finger is then moved towards the camera in the chest and is easily seen. Once the finger has entered the chest cavity, it is replaced by a 10-mm CO2 port. The subxiphisternal port is then used for a hook diathermy, 5-mm SILS endo dissector or 5-mm peanut to clear the thymus from the retrosternal space, with a three-port approach.
Conduct of the thymectomy
With the 30 degree camera in the lower 5-mm port, the other two ports are used to dissect the thymus away from the sternum by following a line just medial to the internal mammary artery (IMA), up to the IMA vein. The thymus and thymic fat are removed from the diaphragmatic surface and the phrenic nerve closest to the camera is released using bipolar cautery. Further specifiers could be provided or 5 mm endoscopic scissors. The thymus is lifted anteriorly and released from the pericardium. A very low threshold exists for obtaining a disc of pericardium with the sample, especially in older patients or larger thymomas where the tumor may be more invasive in nature. If approaching from the left hemithorax, the left IMA (LIMA) vein is followed superiorly and then posteriorly until it inserts into the innominate vein (Figure 5). The thymus is then stripped off the innominate vein as far as is convenient. The left superior horn can also be followed above the LIMA vein. If approaching from the right hemithorax, the thymus is first stripped off the right phrenic nerve (Figure 6). The superior vena cava (SVC) is followed up to the right IMA (RIMA) vein and then across the innominate vein, with clipping of the thymic vein.
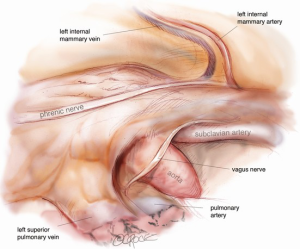
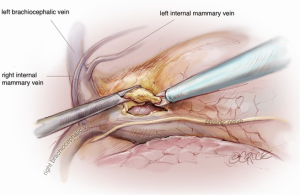
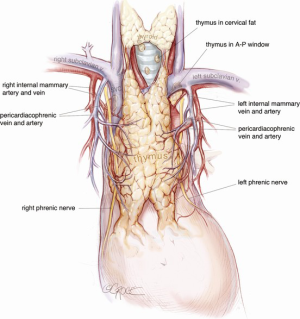
The camera is then changed to the subxiphoid port, which facilitates an excellent view of the opposite phrenic nerve. In the case of an initial left chest approach, instruments are still used from the two left-sided 5 mm ports. One is used to elevate the thymus, and a hook diathermy or energy device is used to remove the thymus from the right phrenic nerve. This plane of dissection is followed up the SVC to the RIMA vein and across to the innominate vein. A 5-mm clip may be applied to the thymic vein, which invariably arises from the mid-point of the innominate vein. Vessels arising from the IMA, internal mammary vein, thyrothymic ligament and inferiorly from pericardiophrenic vessels are usually small enough to be divided by diathermy or an energy device. Finally, the right superior horn is released. Additional tissue under the innominate vein is also removed and the view of this process and of the cervical superior horns from the subxiphoid port is excellent. The key vascular anatomy of the thymus is demonstrated in Figure 7.
Removal of the thymus
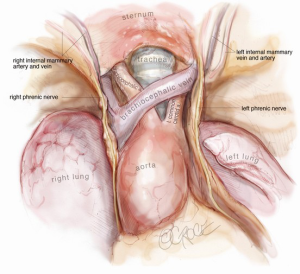
After release of the thymus, it is placed in the left hemithorax, and the area is checked for bleeding. Intercostal nerve blocks are placed using a long needle under vision from the subxiphoid port. The sample is only removed as the final intraoperative step, as CO2 insufflation will be lost once the subxiphoid port is extended to remove the thymus in a retrieval bag. Once removed in a bag, a single size 24 French drain is inserted into the mediastinum. We find that two drains are unnecessary. In addition, the drain establishes the absence of a pneumothorax, as bleeding is invariably minimal with this technique. Once the thymus has been removed in full, one should be able to visualize the remaining anatomy very clearly from the subxiphoid port and fully evaluate the extent of resection (Figure 8).
Safety considerations
Throughout the operation, there is a 5-mm suction irrigator available for use. We also have 5-mm dissecting peanuts and 5- and 10-mm endoclip applicators available. If a larger vessel bleeds, then pressure can be applied and the suction irrigator can be used to identify the source of bleeding, which can then be grasped prior to application of a clip. If the innominate vein is damaged, then it must be remembered that this is a low pressure vessel and the patient can be placed in a reverse Trendelenberg position to further reduce the venous pressure. Additional CO2 insufflation can also collapse small low pressure veins to provide more time to identify the location of bleeding.
Comments
The most important consideration in VATS thymectomy is that the same quality of resection must be achieved as by an open operation. Challenges include adequate visualization of both phrenic nerves and of the superior horns above the innominate vein. If an equivalent resection is achieved as that possible by sternotomy, then the VATS technique will significantly reduce pain compared to sternotomy and speed up recovery. Microthymectomy achieves many of these aims. The subxiphoid port allows an excellent view of both phrenic nerves and the superior horns of the thymus. It also allows painless removal of the sample at the end of the operation. The 5-mm ports allow the surgeon to use a multiport approach that may be more familiar than a uniportal subxiphoid approach. We encourage surgeons to try this approach as one method of performing VATS thymectomy.
Acknowledgements
We are very grateful for the outstanding illustrations provided by Beth Croce.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [PubMed]
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 1999;67:1808-10. [PubMed]
- Mineo TC, Ambrogi V, Paci M, et al. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy. Arch Surg 2001;136:783-8. [PubMed]
- Suda T, Ashikari S, Tochii S, et al. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718-9. [PubMed]
- Levinson MM. Subxiphoid multi-arterial OPCAB: surgical technique and initial case report. Heart Surg Forum 2005;8:E303-10. [PubMed]
- Levinson ML, Fonger J. Minimally invasive atrial septal defect closure using the subxiphoid approach. Heart Surg Forum 1998;1:49-53. [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [PubMed]





