Influence of higher valve gradient on long-term outcome after aortic valve repair
Introduction
Aortic valve repair (AVr) has emerged as a safe, feasible and effective alternative to valve replacement in patients with aortic insufficiency (AI) (1-4). This owes to a number of advances in the field, including better understanding of the functional anatomy of the aortic valve (AV), awareness of the mechanisms that cause AI and the development of a universal classification system that improves clinical practice and research (5-7). The advantages of AVr over traditional valve replacement (AVR) pertain to the preservation of the native aortic valve. This has been shown to reduce long-term valve-related complications and to negate the risk of major haemorrhage associated with lifelong anticoagulation in patients requiring mechanical prostheses (1,2). Concerns do exist, however, regarding the durability of reparative techniques, and freedom from AV re-intervention is therefore an important outcome especially in younger patients with greater life expectancy.
If AVr is to supersede AVR as the treatment of choice in patients with pure AI, then it is necessary to accurately assess repair durability and the factors that contribute to AV re-intervention. Previously, it has been shown that risk factors for AV re-intervention following AVr include younger age (8), bicuspid leaflet morphology (9), commissural orientation (8), use of a pericardial patch (8), aorto-ventricular junction diameter (8), post-operative effective cusp height (8) and the presence of pre-discharge AI (10). To date, no study has considered the impact of post-operative AV gradient on the need for AV re-intervention after AVr. This is despite the fact that higher post-operative AV gradient has been shown to be associated with the need for re-operation after AVR, such as in patient-prosthesis mismatch, acute implant thrombosis or pannus formation (11,12). Higher post-operative gradient may also be a modifiable risk factor in that it may be detectable at the time of surgery with transoesophageal echocardiography (TOE), allowing early correction (13). This study, therefore, aimed to evaluate the impact of raised post-operative AV gradient on freedom from AV re-intervention after AVr, especially in younger patients.
Methods
Study population and investigations
This study comprised a retrospective analysis of prospectively collected data on patients undergoing AVr at a single institution between March 1996 and June 2010. The local institutional review board waived the requirement for participant consent. Patients were included in the study if they had a diagnosis of AI and had undergone AVr including techniques to repair or replace the aortic sinuses, root or ascending aorta. The sole exclusion criterion was patients aged less than 18 years of age at the time of operation. All aortic valve leaflet conformations were permitted. Routine pre-operative investigations included echocardiography to determine the severity of AI, aortic leaflet conformation, left ventricular (LV) function, and cardiac and aortic dimensions. Coronary angiography was also performed in selected patients to evaluate the need for concomitant coronary artery bypass grafting (CABG).
Surgical technique
The surgical technique and principles for tricuspid and bicuspid AVs has been described by our group elsewhere (1,3,7).
Echocardiography
Postoperative echocardiography was performed before discharge to determine the peak AV gradient and to assess potential complications. Patients were divided into two groups on the basis of postoperative peak AV gradient. Group I comprised those with a peak AV gradient <20 mmHg, while Group II included those with a peak AV gradient ≥20 mmHg. We chose 20 mmHg as the cut-off peak gradient as we felt that this may represent the value signifying the beginning of increased turbulence across the valve before ‘stenosis’ develops. After discharge patients were followed up at 6 weeks, 6 months and yearly thereafter.
Data collection and outcomes
Data were collected from bespoke hospital databases maintained prospectively. This included details of participant demographics (age, gender, height, weight, body surface area), pre-operative echocardiographic parameters, operative characteristics (nature and duration of surgical procedure performed, pathological findings, concomitant procedures), early postoperative events (mortality, complications, residual AI and AV gradient) and follow-up details (duration of follow-up, cardiac survival, need for valve re-intervention, echocardiographic parameters).
The primary outcomes were freedom from AV re-intervention, overall survival and cardiac survival. Freedom from AV re-intervention was defined as the time from the day of AVr to the day of any surgical or percutaneous reoperation on the native AV. Overall survival was defined as death from any cause from the date of surgery to final follow-up. Cardiac survival was defined as death from any heart-related cause from the date of surgery to final follow-up, including secondary to any potential valve-related sequelae (e.g., thromboembolism, endocarditis and haemorrhage). Patients lost to follow-up were censored at the date of last contact. Secondary outcomes included in-hospital mortality (defined as death from any cause within 30 days of surgery and/or during the index hospital admission), early complications (<30 postoperative days), late valve-related events (>30 postoperative days) and echocardiographic outcomes (AI grade, AV area, AV gradient, LV function, LV dimensions, aortic dimensions).
Statistical analyses
Statistical analyses were performed using Stata version 11 (StataCorp, College Station, Texas, USA). Data is expressed as mean ± standard deviation or median with range, as appropriate. To compare continuous variables either the student’s unpaired t-test or Mann-Whitney U test were used. For categorical variables, the Chi-squared or Fisher’s exact tests were utilised. The Kaplan-Meier method was used to evaluate time-dependent variables and comparisons were made between groups using the logrank test of equality. Logistic regression analysis was used to determine: (i) pre-operative predictors of early post-operative peak AV gradient ≥20 mmHg; and (ii) independent predictors of AV re-intervention at final follow-up. One subgroup analysis was planned a priori and this examined the demographic and clinical differences between Group I and II patients who underwent AV re-intervention. A P-value of <0.05 was considered statistically significant.
Results
Patients
A total of 471 patients met the eligibility criteria and were included in the study. These were categorised into 358 patients in Group I and 113 in Group II on the basis of early postoperative peak AV gradient, as discussed before. The pre-operative demographic and clinical details of patients are listed in Table 1. The mean age of participants in Groups I and II were 53.6±16.0 and 50.6±16.4 years, respectively (P=0.08). There were 294 males (82.1%) in Group I and 88 males (77.8%) in Group II (P=0.33). Impaired LV function (n=44, 12.2% vs. n=12, 10.6%; P=0.73) and body surface area (1.97 vs. 1.95 m2; P=0.4) were similar between groups. A number of baseline differences existed between the two groups. More patients in Group II had pre-operative AI grade >2+ (n=78, 69.0% vs. n=192, 53.6%; P=0.004), bicuspid valves (BV; n=58, 51.3% vs. n=106, 29.6%; P=0.0001) and restrictive valves (n=34, 30.0% vs. n=52, 14.5%; P=0.0001), while Marfan patients were present only in Group I (n=19; P=0.010). In addition, pre-operative echocardiography demonstrated that patients in Group II had greater left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter, while the dimensions of the aortic sinuses, sino-tubular junction and ascending aorta were generally larger in Group I.
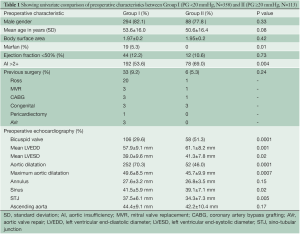
Full table
Operative details
Table 2 summarises the operative details. The mean cardiopulmonary bypass time was 117.0±68.2 minutes in Group I and 105.3±36.2 minutes in Group II (P=0.08). In Group I, the mean aortic cross clamp time was 99.3±41.0 minutes as compared to 82.8±32.7 minutes in Group II (P=0.0001). More valve-sparing, re-implantation and re-modelling procedures were performed in Group I, while there was a greater proportion of ascending aorta replacements and shaving and/or decalcification interventions in Group II. Concomitant procedures were performed in 106 patients in Group I (29.6%) and 42 patients in Group II (36.2%; P=0.4). Amongst these were some 63 mitral valve (MV) repairs, 53 CABGs and 15 tricuspid valve (TV) repairs.

Full table
Early postoperative outcomes
There were two cases of in-hospital mortality in Group I (0.5%) and none in Group II (0.0%; P=1.0). Early complications (<30 days post surgery) are shown in Table 3. There were no cases of endocarditis or cerebrovascular events and no difference in complication rates between groups. Seven patients required early AV re-intervention within 30 days post surgery (Group I: n=5, Group II: n=2; P=0.67). At discharge, no patients had AI grade >2+ in either Group I or II.
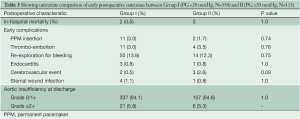
Full table
Late postoperative outcomes
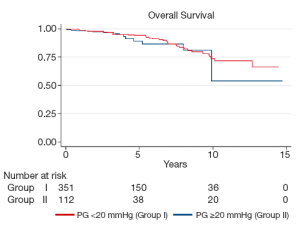
Mean follow-up for Groups I and II was 123.1±89.7 and 147.1±108.0 months (P=0.01), respectively. During this time there were 34 deaths in Group I (9.4%) and 10 deaths in Group II (8.8%; P=1.0). Twenty deaths (5.5%) in Group I and 6 (5.3%) in Group II were cardiac-related (P=1.0). Kaplan-Meier analysis demonstrated similar overall survival between the two groups (log-rank P=0.55). At 5-years, overall survival was 94.1±0.3% in Group I and 89.1±0.4% in Group II, while at 10 years it was 73.6±0.9% in Group I and 54.0±2.4% in Group II (Figure 1). Cardiac survival was also similar between the two groups (logrank P=0.53). At 5 and 10 years it was 96.5±0.2% and 92.0±0.4% and 82.2±0.9% and 89.6±0.5%, in Group I and II, respectively (Figure 2).
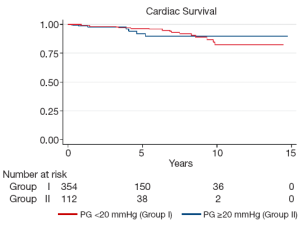
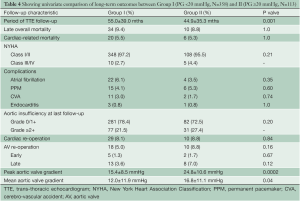
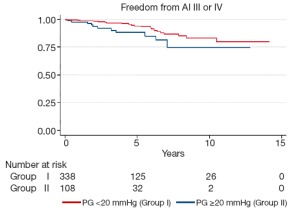
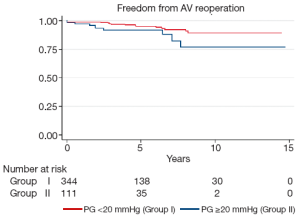
Late complications (>30 days post surgery) are shown in Table 4. Atrial fibrillation, stroke, pacemaker insertion and endocarditis were similar between the two groups. The mean echocardiographic follow-up was 55.0±39.0 months in Group I and 45.2±35.7 months in Group II (P=0.0001). The mean AV gradient at final follow-up was 12.0±11.9 mmHg in Group I compared to 16.8±11.1 mmHg in Group II (P=0.04). At final follow-up 22 patients were AI grade >2+ in Group I (6.1%), while 11 were in Group II (9.7%; P=0.20). Kaplan-Meier analysis demonstrated that freedom from AI >2+ was longer in Group I than in Group II (P=0.03). At 5 and 10 years, freedom from AI >2+ was 94.4±0.3% versus 88.4±0.4%, and 83.6±0.7% versus 74.4±0.9% in Groups I and II respectively (Figure 3). Overall, AV re-intervention was required in 28 patients during follow-up (Group I: n=18, 5.0%; Group: n=10, 8.8%; P=0.16), of which 21 were considered late procedures (performed >30 days after the primary intervention). As determined by Kaplan-Meier analysis, freedom from AV re-intervention was significantly longer in patients in Group I compared to Group II (logrank P=0.02). At 5-years, freedom from AV re-intervention was 95.2±0.3% in Group I while 91.9±0.3% in Group II. Similarly, at 10-years after surgery, freedom from AV re-intervention was 89.2±0.6% in Group I and 76.9±0.9% in Group II (Figure 4). Sub-group analysis showed that Group II patients requiring late AV re-intervention (n=10) were younger (41.8±13.1 vs. 51.0±16.0 years; P=0.08) with a similar proportion of bicuspid valves (n=6; 60%; P=0.74). The reasons for AV re-intervention in this subgroup were recurrent AI (n=7) and combined AI and stenosis (n=3).
Full table
Multivariate analysis
All baseline covariates that differed significantly between the two groups were entered into a logistic regression model to analyse predictors of early postoperative peak AV gradient ≥20 mmHg. This identified calcified, restrictive and bicuspid valves as independent predictors of early postoperative AV gradient ≥20 mmHg (P=0.04 for each). Similarly, a logistic regression model was set up to identify independent predictors of the need for AV re-intervention at final follow-up. This demonstrated that increased preoperative end-diastolic diameter (P=0.03) and younger age (P=0.007), but not PG≥20 mmHg (P=0.98), were independent predictors of AV re-intervention during follow-up.
Discussion
This study investigated the impact of higher early postoperative peak AV gradient on long-term outcome after AVr. Here, we have shown that AVr is safe and effective both in terms of operative morbidity and mortality, long-term survival and freedom from AV re-intervention. It is important to note that pre-operatively, severe AI was greater in the group with the higher post-operative gradient. Moreover, patients with a higher post-operative gradient were more likely to have bicuspid valves and restrictive valves pre-operatively. Intrinsically, this leads one to believe that patients with a higher post-operative gradient had more complex cusp repairs (decalcification and shaving) for associated worse AI and complex valve pathology than those with lower post-operative gradients. This was also borne out from the logistic regression showing that restrictive, calcified and bicuspid valves were more likely to have higher gradients.
We have also demonstrated that patients with higher post-operative peak AV gradients were more likely to have recurrence of AI and require AV re-intervention during follow-up, although their survival was not significantly affected by this. In the regression analysis of the whole cohort, younger patients were more likely to require re-operation. Furthermore, younger patients in the higher gradient group were more likely to require late AV re-intervention than older ones. The reason for this could be three-fold: one, that in patients with a higher gradient, the repair may not hold in the long-term leading to insufficiency. Secondly, the velocity of blood may perpetuate future calcification and stenosis. Thirdly, there may be progression of native disease. All these assume more significance in younger patients as they are likely to live longer. In these circumstances, the peri-operative utilisation of imaging techniques to identify those at risk or the application of surgical techniques that reduce the likelihood of raised transvalvular gradients may be beneficial in reducing the need for AV re-intervention.
Few studies have considered the impact of raised early post-operative AV gradient on long-term outcomes of AVr. Indeed, reviews of the literature undertaken both in the preparation of this manuscript and also by Petterssen et al. (14) found no studies that have investigated the impact of early post-operative transvalvular gradient on the durability of AVr. The latter did, however, hypothesise that raised early post-operative AV gradient following AVr was likely to reduce its durability and freedom from AV re-intervention (14). High pressure across the repair may cause greater stress, prevent healing and lead to LV impairment (15,16). This seems reasonable given that raised transvalvular gradients are common causes of both early and late re-operation following AVR. Such gradients can develop as a result of patient-prosthesis mismatch, acute valve thrombosis or pannus formation. Recently, a study by Riegel et al. (17) considered the impact of raised intra-operative and post-operative transvalvular gradients on early re-operation rate following MV repair. This study showed that mean and peak trans-mitral gradients of greater than 7 and 17 mmHg respectively were associated with the need for MV re-intervention during the same admission. The authors, however, did not report any long-term outcomes.
This is the first study of its kind comparing the effect of higher post-operative valve gradient on freedom from valve re-intervention in patients undergoing AVr. However, it is important to interpret this study in light of its limitations. First, it is a non-randomised, retrospective comparison of two groups of patients and it is possible that baseline differences in covariates and selection bias could have affected the findings. However, the data from which the study was derived was collected prospectively and multivariate analyses performed. Given the lack of well-designed comparative studies and the excellent modern outcomes for AVr, a randomised multi-centre trial of AV repair versus AV replacement along with a sub-group analysis of high post-operative gradient patients seems warranted. This may be methodologically difficult, however, because of heterogeneity in the techniques and the lack of specialist centres with sufficient experience in the nuances of AVr. In summary, this study has shown that raised early post-operative AV gradient after AVr may be associated with worse freedom from AV re-intervention. Strategies to limit the development of the gradient and to detect its occurrence early are warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg 2010;140:276-84.e1.
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32.
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94.
- Ashikhmina E, Sundt TM 3rd, Dearani JA, et al. Repair of the bicuspid aortic valve: a viable alternative to replacement with a bioprosthesis. J Thorac Cardiovasc Surg 2010;139:1395-401.
- Underwood MJ, El Khoury G, Deronck D, et al. The aortic root: structure, function, and surgical reconstruction. Heart 2000;83:376-80.
- Fedak PW, Verma S, David TE, et al. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002;106:900-4.
- Boodhwani M, El Khoury G. Principles of aortic valve repair. J Thorac Cardiovasc Surg 2010;140:S20-2; discussion S45-51.
- Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85.
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32.
- Sareyyupoglu B, Suri RM, Schaff HV, et al. Survival and reoperation risk following bicuspid aortic valve-sparing root replacement. J Heart Valve Dis 2009;18:1-8.
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000;36:1131-41.
- Lawton JS, Moazami N, Pasque MK, et al. Early stenosis of Medtronic Mosaic porcine valves in the aortic position. J Thorac Cardiovasc Surg 2009;137:1556-7.
- Nowrangi SK, Connolly HM, Freeman WK, et al. Impact of intraoperative transesophageal echocardiography among patients undergoing aortic valve replacement for aortic stenosis. J Am Soc Echocardiogr 2001;14:863-6.
- Pettersson GB, Crucean AC, Savage R, et al. Toward predictable repair of regurgitant aortic valves: a systematic morphology-directed approach to bicommissural repair. J Am Coll Cardiol 2008;52:40-9.
- Grande KJ, Cochran RP, Reinhall PG, et al. Mechanisms of aortic valve incompetence in aging: a finite element model. J Heart Valve Dis 1999;8:149-56.
- Deck JD, Thubrikar MJ, Schneider PJ, et al. Structure, stress, and tissue repair in aortic valve leaflets. Cardiovasc Res 1988;22:7-16.
- Riegel AK, Busch R, Segal S, et al. Evaluation of transmitral pressure gradients in the intraoperative echocardiographic diagnosis of mitral stenosis after mitral valve repair. PLoS One 2011;6:e26559.





