Novel mitral valve technologies—transcatheter mitral valve implantation: a systematic review
Introduction
Valvular heart disease is an important cause of morbidity and mortality throughout the world; in industrialized nations, which have a typical low rate of rheumatic valve disease, mitral regurgitation (MR) is the most common valvular lesion (1). Untreated, severe MR has a poor prognosis, with a 5-year mortality rate of up to 50% (2). Treatment of MR depends on its pathophysiology. Primary MR results from dysfunction of the mitral valve apparatus whereas secondary MR occurs in patients with an alteration in the left ventricle geometry causing a failure of coaptation of a structurally normal mitral valve.
Surgical repair of symptomatic, severe primary MR has been demonstrated to improve survival (3) and this is reflected in current guidance for treatment (4,5). However, for the treatment of secondary MR, the benefit of surgical intervention is less clear. Surgical repair is associated with significant morbidity and mortality, meaning there is a large proportion of patients for whom the risk of conventional treatment is prohibitive. This group of patients often have multiple co-morbidities or previous surgery. The development of transcatheter technologies provides a potential option for patients not suitable for conventional valve surgery. The mitral annulus is a complicated structure, with a non-uniform shape that makes the development of transcatheter valve replacement difficult. Another major factor that is considered prior to implanting a transcatheter mitral valve is the risk of inducing left ventricular outflow tract obstruction (LVOTO). Current use for transcatheter mitral valve replacement for native valve disease has predominantly been in the setting of secondary mitral regurgitation. The treatment of primary MR has seen the development of transcatheter technologies including edge-to-edge-repair, transcatheter neo-cord implantation and transcatheter annulus reinforcement. The difficulty in developing an optimal system for the replacement of the mitral valve can be seen in the heterogeneity of the devices currently undergoing early clinical trials.
This review aims to assess the early outcomes of newly developed transcatheter mitral valve implantation technologies for the treatment of secondary native valve disease. Furthermore, the outcomes of patients receiving transcatheter treatment of regurgitant failure of surgically repaired or replaced mitral valves has also been addressed. Post-surgical failure with regurgitation is associated with an increased risk of mid-term mortality compared with failure by stenosis (6), therefore patients with post-surgical failure by stenosis are not assessed in this analysis.
Methods
This systematic review was performed in accordance with PRISMA recommendations and guidance. The search strategy was employed to search electronic databases EMBASE, Ovid Medline, the entire Cochrane Central Register of Controlled Trails (CCRCT), Cochrane Database of Systematic reviews (CDSR), the Database of Abstracts of Reviews of Effects (DARE) and the ACP journal club from their inception to September 2017. The search strategy included search terms for (mitral valve replacement OR mitral valve implantation OR tmvr OR tmvi) AND transcatheter. The bibliography of previous systematic reviews was assessed to ensure no additional missed publications.
Selection criteria
Eligibility for inclusion in this systematic review included studies which assessed the outcomes of patients undergoing transcatheter mitral valve implantation for patients with secondary mitral regurgitation and patients with regurgitant failure of previous mitral valve repair or bioprosthetic failure treated with transcatheter mitral valve-in-valve (ViV) or valve-in-ring (ViR) implantation. When centers reported outcomes of overlapping patient series, then the most contemporary series was analyzed. The analysis was limited to English language papers. Conference abstracts, case reports, editorials, expert opinion, reviews and expert opinion were excluded, as were patients undergoing combined valvular interventions in the same procedure. Individual case reports have been included in the assessment of valve-in-valve and valve-in-ring procedures, given the paucity of published data.
Data extraction
For the assessed papers, data was extracted from the reviewed text, tables and figures. Data was extracted independently by the authors and any discrepancies were reviewed and discussed until consensus was reached. The recorded parameters were: number of cases in series, procedure undertaken, pre- and post-operative New York Heart Association (NYHA) score, average age, average follow up, early death, late death, timing of death, late bleeding complications, systemic thromboembolic complications including valve thrombosis, post-operative endocarditis, hospital length of stay, circulatory mechanical assist support, para-valvular leak and presence of hemolysis, pre- and post- operative mean mitral gradient, pre-and post-operative mitral regurgitation grading and emergency reoperation.
Results
The search strategy revealed 1,127 records after duplications were removed, 25 of which met pre-determined inclusion criteria. Of this 25, nine studies were related to transcatheter mitral valve replacement (TMVR) in secondary MR and 16 studies were related to ViV or ViR TMVR for regurgitant failure of prior surgical repair/replacement. See Table S1.
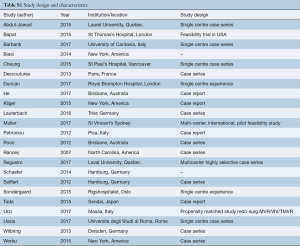
Full table
Transcatheter mitral replacement for secondary mitral regurgitation
The nine studies included a total of 112 patients with a mean age of 72.7±4.4 years and a mean follow-up duration of 226.9±287.6 days. There were 77 male patients and 35 female patients, 81% of patients (91 patients) had a NYHA score of greater than or equal to 3. Patients in all studies were at a significant risk for operative intervention. The mean Society of Thoracic surgeons (STS) score was 15.1%±11.0% and the mean EuroSCORE was 20.4%±13.4%. The studies reported the results of six different systems (Edwards Fortis valve, Abbot Tendyne valve, Neovasc Tiara valve, Medtronic Intrepid valve, Edwards CardiAQ valve and the Highlife valve). The most common reported delivery route was transapical, which was used for 98.2% of patients (110 patients). Overall, there were 24 deaths (21.4%) during follow up with 15 (13.4%) early deaths (in same hospital admission or Table 1.
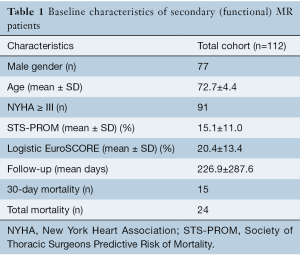
Full table
Edwards Fortis valve
Two manuscripts assessed the outcomes of the Edwards Fortis system (7,8). These two manuscripts included 16 patients with a mean age of 71 and a mean follow-up duration of 456±387.5 days. The mean STS score was 8.3±1.5 and the mean EuroSCORE was 25.6±2.7. All patients had the transcatheter mitral valve delivered via transapical access. There were 12 successful valve deployments [according to the mitral valve academic research consortium (MVARC) consensus statement (9)] and two failed deployments, both of whom subsequently underwent surgical mitral valve replacement and died in the early post-operative period. In total, there were five early deaths (31%) (three peri-procedural and two within 30 days) and two further deaths within the follow-up period. The surviving patients demonstrated sustained improved heart failure symptoms with NYHA II in all but one patient. There was no left ventricular outflow tract obstruction (LVOTO), no residual MR >1+ and a mean trans-mitral gradient of 2.8±0.7 mmHg. A high rate of valve thrombosis in three patients (19%) led cessation of implantation of this valve in 2016.
Abbott Tendyne valve
Two manuscripts assessed the outcomes of the Abbott Tendyne system (10,11), including a total of 35 patients with a mean age of 74.5±1.6 years and a mean follow-up duration of 361.5±469.1 days. The 30 patients included in the manuscript of Muller et al. had early outcomes at 30 days assessed. The mean STS score was 11.4±5.7 and the mean EuroSCORE was 11.7±7.3. The Tendyne system delivers the prosthesis via a transapical approach. There were 32 (91%) technically successful device implants and there were 3 failed deployments [one patient had a larger prosthesis successfully deployed (10)] and one patient required peri-procedural mechanical support due to LVOTO which was subsequently treated successfully with a left ventricular outflow tract (LVOT) stent (11). Overall, there were two deaths (6%), one early death and one late death. The majority of patients had an improvement in heart failure symptoms with 25 patients (71%) being NYHA I or II. In all of the surviving patients with a successfully implanted Tendyne valve there was no or mild para-valvular regurgitation and no transvalvular regurgitation. The mean transvalvular gradient was 3.3±0.1 mmHg. Two patients (6%) developed valve thrombosis, one of which resolved with anticoagulation, one patient (3%) had a stroke and there was one (3%) episode of endocarditis. Two patients (6%) developed hemolysis.
Neovasc Tiara valve
One published manuscript assessed the early outcomes of the Neovasc Tiara system (12), including a total of two patients with a mean age of 67±8.5 years and a mean follow-up duration of 65 days. The mean STS score was 26.1±30.5 and the mean EuroSCORE was 16.9±11.0. Via a transapical route, both patients had a technically successful implantation of a Tiara valve with no residual MR. There was one death which occurred at 69 days due to recurrent, refractory heart failure after initial discharge from hospital. The surviving patient had a mean transmitral gradient of 2 mmHg and was NYHA II at 2-month.
Medtronic Intrepid valve
One manuscript assessed the outcomes of the Medtronic Intrepid Valve (13), including a total of 50 patients with a mean patient age of 72.6±8.8 years and a mean follow-up duration of 261.7±229 days. The mean STS score was 6.4±5.5 and the mean EuroSCORE was 7.9±6.2. All patients had the valve delivered via a transapical route. There were 48 technically successful implants in the patient population, eight patients (16%) required mechanical assist devices [five patients received an intra-aortic balloon pump and three patients received extra-corporeal membrane oxygenation (ECMO) support]. There were seven early deaths (14%) and four subsequent deaths on longer term follow-up, three deaths were related to apical access site bleeding, one patient with a malpositioned valve causing and three patients were a result of refractory heart failure. For all surviving patients the MR was grade
Edwards CardiAQ valve
Two manuscripts assessed the outcomes of the Edwards CardiAQ valve (14,15), including a total of seven patients with a mean age of 76.6±5.1 years and a mean follow-up duration of 226±150 days. The mean STS score was provided by Sondergaard et al. 34.4±11.2 (15) and the mean EuroSCORE provided by Ussia et al. was 39.7±2.4 (14). The valve was delivered transapically for four patients and via a transfemoral, transseptal approach in three patients. All valve deployments were technically successful. There was one early death (14%) at 8 days and two late deaths (28%). There was no LVOTO, no valve embolism, hemolysis or endocarditis; one patient developed valve thrombus which resolved with anticoagulation. The mean ICU length of stay (0.25±0.4 versus 3.2±2.6 days) and hospital length of stay (7.5±4.9 versus 14.75±3.9 days) was shorter for the patients treated with a transfemoral approach. No patients had residual MR >1+ and all patients had a functional improvement with an NYHA class of I or II on follow-up.
Highlife valve
A single manuscript reports the outcomes of two patients with the highlife valve (16). The patients age was 67±2.8 years with and mean follow-up duration of 78±105 days. The mean EuroSCORE was 4.5±6.7. Both patients received technically successful valve deployments with no residual regurgitation. However, there was one early death (50%) on Day 4 due to refractory cardiogenic shock. There was no LVOTO, valve embolism, valve thrombosis or hemolysis. One patient developed new mild-to-moderate AR post procedure, likely related to injury to the aortic valve cusps when siting the sub-annular component of the valve system. The mean mitral gradient post procedure was 5 mmHg and there was a sustained improvement in functional class for the surviving patient to NYHA II.
Valve-in-valve procedures
There were 11 manuscripts reporting the outcomes of the transcatheter management of failed mitral valve bioprostheses encompassing 44 patients (16-26). The mean age was 79.2±5.4 years and the mean follow-up duration was 197.5±156.5 days. The mean STS score derived from eight reports (16-18,22-26) was 26.1±27.3 and the mean EuroSCORE derived from eight studies (18-24,26) was 42.8±11.1. The mean age of bioprosthesis failure was 12.2±4.6 years. The most commonly placed valve was the Edwards Sapien XT valve with 32 implants (74%) followed by the Edwards Sapien 3 valve which was implanted in six patients (14%), the Edwards Sapien valve was implanted in four patients (9%), one patient (2%) had a Boston Scientific Lotus valve implanted. The most common implant approach was transapical with 40 patients (91%) followed by the transfemoral, transseptal approach which was used for three patients (7%) with one (2%) patient being treated with a transjugular, transseptal approach. The mean post-operative gradient was 5.4±3.0 mmHg. There were three early deaths (7%) and total mortality of 10 patients (23%) at a mean of 163.9±223.6 days post-operatively. The average hospital length of stay was 15.4±15.1 days. One patient required emergency cardiac surgery to salvage an embolized prosthesis, who later died (19).
Valve-in-ring procedures
Five papers described reported transcatheter mitral valve-in-ring procedures encompassing 20 patients (22,27-30). The mean patient age was 63.6±10.3 years with a mean follow-up duration of 126.4±158.0 days. The mean STS score was 20.0±10.6 and the mean EuroSCORE was 40.7±1.8. Repair failure occurred at an average of 6.8±5.2 years. The most common route of implantation was transapical with 13 transapical implants (65%), there were six transfemoral-transseptal implants (3%) and one rail technique (transfemoral-transseptal combined with transapical) (4%). The most commonly implanted valve was an Edwards Sapien XT valve with 17 implants (80%) followed by the Boston Scientific lotus valve which was implanted in two cases (10%). The Medtronic Melody valve and the Edwards Sapien valve were both implanted once (5% each). Post-procedure there were six patients (30%) with grade 2 or greater MR. The mean post procedure trans-mitral gradient was 4.8±0.5 mmHg. There were three early deaths (15%), two patients died of refractory heart failure (27) and one patient died multiple organ failure possibly as a result of emboli (29) of there were an additional two late deaths (22). See Table 2.
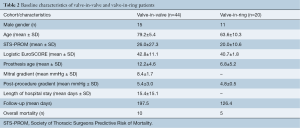
Full table
Discussion
This review has highlighted the early outcomes of the published cases of dedicated transcatheter mitral systems for native regurgitant valve disease the management of failed previous surgical repair. The outcomes generated from early device outcomes demonstrated in this review show a significant mortality risk mortality for both native MR (early mortality 21.4%) and for failed previous mitral surgery (15–23% mortality) in a group of patients who are highly symptomatic but are at prohibitive risk for traditional surgery.
Mitral valve disease is a highly heterogenous disorder and the mitral valve is a remarkably complicated structure. This is reflected in the variety of solutions undergoing development and testing for transcatheter mitral valve replacement. Challenges lie in: (I) delivery systems; (II) valve sizing; (III) prevention of paravalvular leak; (IV) prevention of LVOTO; (V) valve thrombosis; (VI) weighing up the variations between the devices.
The majority of novel transcatheter mitral systems support transapical delivery due to the large prosthesis size and due the significant angulation necessary in a tight space required for a transseptal delivery system. Currently, only the CardiAQ valve is available for a transseptal implant. A transseptal approach may well be desirable by obviating the need for a thoracotomy and minimizing risk of access site bleeding, an important cause of re-intervention and mortality. Furthermore, the early experience shown by Ussia et al. demonstrated a shorter hospital and ICU length of stay from a transfemoral, transseptal implant compared with a transapical implant (14), although significantly more experience is required to ensure there is no compromise on valve position and implant-related complications. Extrapolating the experiences of transcatheter aortic valve implantation experiences avoiding transapical access may reduce peri-procedural mortality and morbidity (31-33).
Valve sizing is additionally of critical importance and a multi-modal approach has been shown to be of utmost importance. Current recommendations include pre-procedural as well as procedural transesophageal echocardiography (TEE) to diagnose severity and mechanism of MR as well as electrocardiogram (ECG)-gated computed tomography (CT) of the chest. Imaging allows the determine annular size and presence of mitral annular calcification, anatomy of the subvalvular apparatus and determine the risk of inducing LVOTO (34). Accurate valve sizing has been shown to be an important factor on implant with at least one death resulting in valve malpositioning being attributed to a miscalculation in valve sizing (13). Both prosthesis and patient anatomy factors determine the risk of LVOTO which is a feared complication both for TMVR in native valve disease and ViV/ViR procedures as the prosthesis extends beyond the mitral annulus into the left ventricle which may depress the anterior mitral valve leaflet or bioprosthetic valve leaflets into the LVOT. ECG-gated CT allows a risk assessment for LVOTO with a simulated valve implant to determine the size of the “neo-LVOT” (35).
Valve thrombosis is a serious issue and, as yet, there is no consensus on the anticoagulation management of this cohort of patients with antiplatelets. Valve thrombosis resulted in the cessation of implantation of the Edwards Fortis valve in 2016 and was noted in 19% of patients with a Fortis valve implantation at 2-year in this review (7,8). There were 2 valve thrombosis (6%) with the for patients implanted with the Tendyne valve one in the context of subtherapeutic INR (10) and a second patient who developed thrombus on the valve after pre-procedural cessation of anticoagulation (11), both resolved with therapeutic anticoagulation. The CardiAQ valve had one valve thrombosis detected at 3-month post-implant [one thrombosis in seven implants (14%)] in a patient who had a contra-indication for systemic anticoagulation (14). There was no reported incidence of implant thrombosis in ViV or ViR implants.
There are a number of distinct variations that exist across the devices, primarily with respect to their ability to be recaptured, how they are tethered and anchored and how they align (i.e., whether circular or D-shaped). The Edwards Fortis valve is non-recapturable, tethered via the subvalvular apparatus and is circularly oriented. The Abbott Tendyne valve is a fully recapturable system after complete deployment, relies on an apical tether—which also aids in sealing the apical access site—and is D-shaped with an outer stent and inner circular frame. It is fixed intra-annularly, with frame sizes ranging from 30–43 mm in the SL dimension and 34–50 mm in the IC dimension. The Neovasc Tiara is non-recapturable, tethered via a fibrous trigone capture with native leaflet engagement and is D-shaped. It comes in a 35 and 40 mm configuration, with two anterior and one posterior anchoring structures. The Medtronic Intrepid is non-recapturable and is anchored via radial force and subannular cleats combined with a “champagne-cork shape” (wider body than neck), which resist valve migration during systole. It comes in a 27 mm inner ring configuration, with 43, 46 and 50 mm outer stent sizes. This dual stent configuration allows for increased fixation and isolation of the inner stent. The CardiAQ-Edwards device is non-recapturable, anchored via mitral annulus capture with native leaflet engagement and is circular shaped. As noted previously, it is unique in that it can be implanted via the transapical-transseptal route. It comes in a 30 mm configuration, sits supra-annularly with an intra-annular sealing skirt and features tapered outflow. The Highlife valve is non-recapturable, externally anchored (valve in subannular mitral ring) and is D-shaped. All valve leaflets are either bovine or porcine trileaflet pericardial tissue. The complex anatomy and heterogeneity amongst this group of patients suggest that none of these solutions will be suitable for all patients. ViV and ViR procedures rely on the radial force exerted by the balloon expandable implant on the previously surgically sited prosthesis.
The current evidence is limited by very small numbers and the short duration of follow-up. The novel devices are all currently enrolling in feasibility and safety studies, which will inevitably result in improvement in device design in this exciting technology. Drawing accurate conclusions from published data for ViV and ViR procedures is more difficult with very poor-quality data being drawn from case reports and small case series. However, this is likely to improve as global experience with these technologies increases. Beyond the development of safe technology, appropriate selection of patients for TMVR is also yet to be established. There is uncertain benefit to the surgical repair or replacement of secondary MR and as seen by the high mortality in the small number of patients included in this review, the benefit of less invasive technologies is yet to be determined.
Conclusions
Transcatheter mitral valve implantation represents a new evolution in the management of valvular disease and affords a treatment option to patients who historically may not have been offered treatment. The complexity of the anatomy and associated abnormal ventricular physiology involved present a greater set of challenges than previously experienced when developing other transcatheter valve implants. Early results have demonstrated promise and with future improvements in technology, imaging modalities and improved patient selection will likely see an improvement in patient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and Outcomes of Unoperated Patients With Severe Symptomatic Mitral Regurgitation and Heart Failure: Comprehensive Analysis to Determine the Potential Role of MitraClip for This Unmet Need. J Am Coll Cardiol 2014;63:185-6. [Crossref] [PubMed]
- Samad Z, Kaul P, Shaw LK, et al. Impact of early surgery on survival of patients with severe mitral regurgitation. Heart 2011;97:221-4. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Yoon SH, Whisenant BK, Bleiziffer S, et al. Transcatheter Mitral Valve Replacement for Degenerated Bioprosthetic Valves and Failed Annuloplasty Rings. J Am Coll Cardiol 2017;70:1121-31. [Crossref] [PubMed]
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial Experience of Transcatheter Mitral Valve Replacement With a Novel Transcatheter Mitral Valve: Procedural and 6-Month Follow-Up Results. J Am Coll Cardiol 2015;66:1011-9. [Crossref] [PubMed]
- Regueiro A, Ye J, Fam N, et al. 2-Year Outcomes After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv 2017;10:1671-8. [Crossref] [PubMed]
- Stone GW, Adams DH, Abraham WT, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 2015;66:308-21. [Crossref] [PubMed]
- Muller DWM, Grayburn PA, Stoler RC, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: a Global Feasibility Trial. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Duncan A, Daqa A, Yeh J, et al. Transcatheter mitral valve replacement: Long-term outcomes of first-in-man experience with an apically tethered device - A case series from a single centre. EuroIntervention 2017;13:e1047-57. [Crossref] [PubMed]
- Cheung A, Webb J, Verheye S, et al. Short-term results of transapical transcatheter mitral valve implantation for mitral regurgitation. J Am Coll Cardiol 2014;64:1814-9. [Crossref] [PubMed]
- Bapat V, Duffy SJ, Reardon MJ, et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12-21. [Crossref] [PubMed]
- Ussia GP, Cammalleri V, Mehta JL, et al. Transcatheter mitral valve replacement with a novel self-expandable prosthesis: Single institutional experience Procedural outcomes and follow-up. J Cardiovasc Med (Hagerstown) 2017;18:415-24. [Crossref] [PubMed]
- Sondergaard L, Brooks M, Ihlemann N, et al. Transcatheter mitral valve implantation via transapical approach: an early experience. Eur J Cardiothorac Surg 2015;48:873-7; discussion 877-8. [Crossref] [PubMed]
- de Biasi AR, Wong SC, Salemi A. Reoperative "valve-in-valve" transapical transcatheter mitral valve replacement in a high-risk patient with a recent transapical transcatheter aortic valve replacement and a degenerated bioprosthetic mitral valve. J Thorac Cardiovasc Surg 2014;148:e209-10. [Crossref] [PubMed]
- Ranney DN, Williams JB, Wang A, et al. Valve-in-Valve Transcatheter Valve Implantation in the Nonaortic Position. J Card Surg 2016;31:282-8. [Crossref] [PubMed]
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012;5:341-9. [Crossref] [PubMed]
- Murzi M, Berti S, Gasbarri T, et al. Transapical transcatheter mitral valve-in-valve implantation versus minimally invasive surgery for failed mitral bioprostheses. Interact Cardiovasc Thorac Surg 2017;25:57-61. [Crossref] [PubMed]
- Poon KK, Clarke A, Luis SA, et al. First Australian Transapical Mitral Valve-in-Valve Implant for a Failed Mitral Bioprosthesis: How To Do It. Heart Lung Circ 2012;21:737-9. [Crossref] [PubMed]
- He C, Scalia G, Walters DL, et al. Transapical Transcatheter Mitral Valve-in-Valve Implantation Using an Edwards SAPIEN 3 Valve. Heart Lung Circ 2017;26:e19-21. [Crossref] [PubMed]
- Schafer U, Bader R, Frerker C, et al. Balloon-expandable valves for degenerated mitral xenografts or failing surgical rings. EuroIntervention 2014;10:260-8. [Crossref] [PubMed]
- Schaefer U, Conradi L, Lubos E, et al. First-in-man treatment of a degenerated mitral surgical valve with the mechanical expanding Lotus valve. EuroIntervention 2016;12:515-8. [Crossref] [PubMed]
- Tada N, Enta Y, Sakurai M, et al. Transcatheter valve-in-valve implantation for failed mitral prosthesis: the first experience in Japan. Cardiovasc Interv Ther 2017;32:82-6. [Crossref] [PubMed]
- Worku B, De Biasi AR, Gulkarov I, et al. Transapical mitral valve-in-valve implantation for patients in cardiogenic shock. Ann Thorac Surg 2015;99:e103-5. [Crossref] [PubMed]
- Wilbring M, Alexiou K, Tugtekin SM, et al. Transapical transcatheter valve-in-valve implantation for deteriorated mitral valve bioprostheses. Ann Thorac Surg 2013;95:111-7. [Crossref] [PubMed]
- Descoutures F, Himbert D, Maisano F, et al. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg 2013;44:e8-e15. [Crossref] [PubMed]
- Kliger C, AlBadri A, Wilson S, et al. Successful first-in-man percutaneous transapical-transseptal Melody mitral valve-in-Ring implantation after complicated closure of a para-annular ring leak. EuroIntervention 2014;10:968-74. [Crossref] [PubMed]
- Lauterbach M, Sontag B, Paraforos A, et al. Transcatheter valve-in-ring implantation of a repositionable valve system for treatment of severe mitral regurgitation. Catheter Cardiovasc Interv 2016;88:E183-90. [Crossref] [PubMed]
- Petronio A, Giannini C, De Carlo M, et al. Antegrade percutaneous valve implantation for mitral ring dysfunction, a challenging case. Catheter Cardiovasc Interv 2012;80:700-3. [Crossref] [PubMed]
- Kumar N, Khera R, Fonarow GC, et al. Comparison of Outcomes of Transfemoral Versus Transapical Approach for Transcatheter Aortic Valve Implantation. Am J Cardiol 2018;122:1520-6. [Crossref] [PubMed]
- Panchal HB, Ladia V, Amin P, et al. A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events in patients undergoing transfemoral versus transapical transcatheter aortic valve implantation using edwards valve for severe aortic stenosis. Am J Cardiol 2014;114:1882-90. [Crossref] [PubMed]
- Hemmann K, Sirotina M, De Rosa S, et al. The STS score is the strongest predictor of long-term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact Cardiovasc Thorac Surg 2013;17:359-64. [Crossref] [PubMed]
- Blanke P, Naoum C, Webb J, et al. Multimodality Imaging in the Context of Transcatheter Mitral Valve Replacement: Establishing Consensus Among Modalities and Disciplines. JACC Cardiovasc Imaging 2015;8:1191-208. [Crossref] [PubMed]
- Blanke P, Naoum C, Dvir D, et al. Predicting LVOT Obstruction in Transcatheter Mitral Valve Implantation: Concept of the Neo-LVOT. JACC Cardiovasc Imaging 2017;10:482-5. [Crossref] [PubMed]






