Safeguards and pitfalls for Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction during transcatheter aortic valve replacement—the BASILICA technique
Introduction
Coronary artery obstruction in the setting of TAVR in native aortic valves is a rare (0.7% incidence) but devastating complication with a 41% thirty-day mortality (1). TAVR for degenerative bioprosthetic surgical valves (Valve-in-valve) carries higher risk still with an incidence of at least 2.3%, and for TAV-in-TAV potentially as high as 20% (2-5). We developed the Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) procedure to prevent coronary artery obstruction in patients deemed to be high risk of coronary obstruction (6). In the following review we summarize predictors of and screening for patients at high risk of coronary artery obstruction, BASILICA procedural steps for single and double leaflet laceration, recent adjunctive techniques, troubleshooting and future directions.
Operative techniques
Preparation
Case selection and procedure planning
BASILICA prevents coronary obstruction in patients at high risk for direct (ostial obstruction) or indirect (sinus sequestration) obstruction. Criteria for selecting patients at high risk for obstruction have been proposed and are including in Table 1.
Table 1
| Criterion | High risk features |
|---|---|
| Native or bioprosthetic aortic leaflet length | Extends above the coronary ostia or above the sino-tubular junction |
| Coronary artery height (measured from the aortic annulus to the lowest part of the coronary artery ostium) | ≤10 mm |
| Virtual valve to coronary artery distance (VTC) (measured in axial plane from edge of virtual ‘valve’ to the center of the coronary artery ostium) | ≤4 mm |
| Virtual valve to sinotubular junction distance (VTSTJ) (measured in axial plane from edge of virtual ‘valve’ to STJ) | ≤2 mm |
| Degenerative bioprosthetic valve | Externally mounted leaflets |
| Stentless surgical valves |
Computed tomography (CT) should be performed in all cases to identify high risk anatomic features and obtain orthogonal fluoroscopic projections that can be used during the BASILICA procedure (3,7). Importantly, if it is not possible to position the C-arm to achieve either CT predicted angle, craniocaudal angulation should be reduced whilst maintaining coronary artery alignment to the side or center for accurate catheter positioning and subsequent leaflet traversal.
BASILICA procedure
Operation
Single (“solo”) Leaflet BASILICA
Solo BASILICA procedures are performed most commonly (8). Procedural steps are outlined in Figures 1 and 2. Right leaflet BASILICA is more technically challenging than left because optimal fluoroscopic angles are frequently difficult to achieve and catheter positioning is tricky.

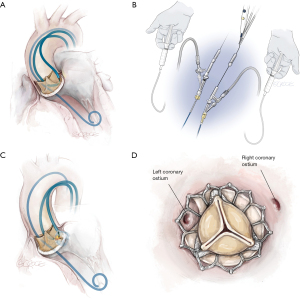
Double (“Doppio”) Leaflet BASILICA
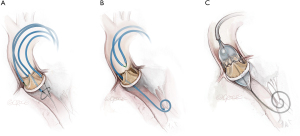
Doppio leaflet laceration (Figure 3) combines laceration of left and right aortic leaflets. Technical complexity is increased with a greater number of procedural steps, multiple interacting and overlapping catheters, longer procedure times and increased contrast volumes. Following laceration of the second leaflet, THV deployment needs to be more expeditious than for solo procedures. For these reasons it is recommended that operators only undertake a doppio when they have developed competence with solo BASILICA, generally after two to three procedures. In the multi-center International BASILICA Registry, procedural success and adverse events including stroke, death and VARC-2 safety outcomes were not significantly different between doppio and solo procedures in experienced hands (8).
Balloon-Augmented (BA) BASILICA
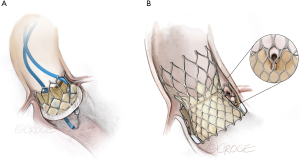
Balloon augmentation (Figure 4) is a recent adjunctive technique that further enlarges leaflet splay area when performed in vitro. Following balloon-augmented laceration a rounded expansion towards the leaflet base enables a wider splay (9). BA-BASILICA has been reported in patients predicted to be at extreme risk of coronary artery obstruction despite conventional BASILICA, including TAV-in-TAV cases. BA-BASILICA was technically successful in all patients. When coronary artery stenting was required it was possible in a conventional, orthotopic fashion.
Completion
Troubleshooting
Meticulous adherence to the described procedural steps permits uncomplicated BASILICA in the majority of cases. Procedural cadence can be interrupted in several ways, outlined below, which have a common theme—failure to concentrate electrosurgical energy during leaflet traversal or laceration.
Failure to traverse or lacerate
Connections between electrosurgical generator and pencil should be checked to ensure it is inserted correctly and that it is connected to the cut function (yellow), not coagulation function (blue). The proximal end of the guidewire should be denuded effectively to remove all insulative polytetrafluoroethylene (PTFE) (green) coating. Cross clamping of the guidewire to the electrosurgical pencil should be secure—the wire should not move from the jaws of the hemostat when tugged. The second operator should be aware to press the yellow “cut” button during application of energy, not the blue “coagulation” button. Energy should be appropriately selected—generally 30–50 W for leaflet traversal and 70 W for leaflet laceration.
Failure to traverse
Catheter positioning on the surgical aortic valve sewing ring may be misinterpreted as appropriate placement. Subsequent attempts at wire traversal will fail as it advances in to the ring rather than across leaflet tissue. Visual cues that may differentiate ring from leaflet engagement include an undulation of the catheter tip (moving synchronously with the annulus) suggestive of sewing ring engagement as opposed to bouncing (paradoxical movement of the catheter tip and target leaflet). If encountered, the traversal guide catheter should be repositioned until it bounces or it may require to be ‘upsized’ (e.g., from AL2 to AL3).
Care should be taken to ensure that the coaxial system of guiding catheter, microcatheter and guidewire is positioned orthogonal to the base of the target leaflet prior to attempted traversal. If this is not achieved the wire may be visualized ‘skidding’ tangentially over the leaflet surface towards the annulus.
Dense calcification of the leaflet base at the intended crossing point may prevent energy transfer and vaporization of tissue. The wire will deflect and should be repositioned. Cases with dense, confluent calcification at the leaflet base may not be appropriate for BASILICA procedures.
Failure to lacerate
When charge concentration is adequate, steady maintenance of gentle traction will result in leaflet laceration. If charge fails to concentrate the operator should avoid increasing force using a ‘lawnmower pull cord’ maneuver and instead halt and evaluate the system concentrating on the following areas.
The displacement of ionic blood by non-ionic 5% dextrose permits effective charge concentration at the lacerating surface by removing alternative current paths (10). It is also beneficial in preventing accumulation of char and thrombus at the cutting surface. The operator should ensure all 3-way connectors are open to the catheter and that rate of flush is satisfactory.
When applying traction to the catheter systems the operator should be vigilant to the positioning of the insulating microcatheters, displacement of which will allow for current dispersal (10). If microcatheter slippage is observed, energy delivery should be stopped immediately. The microcatheter locking mechanism should be inspected to ensure it is closed in the lock position. All torquers should be adjusted to a position of close apposition with microcatheter, wire or rotating hemostatic valve as appropriate and re-tightened.
Comments
Clinical results
The BASILICA IDE trial reported thirty-day and one-year results in a cohort of thirty prohibitive risk patients with multiple comorbidities undergoing BASILICA (11,12). Procedural success was reported in twenty-eight patients, disabling stroke in one and no early or late coronary obstruction. Real world data from the international BASILICA registry including 214 patients from twenty-five centers demonstrated that BASILICA was feasible with successful leaflet traversal and laceration in 94.4%. Doppio procedures were performed in 21.5%. At thirty days disabling stroke was reported in only 0.5%, 95.3% had freedom from culprit coronary artery obstruction and survival was 97.2%.
Advantages
Alternative approaches to prevent or treat coronary artery obstruction are surgery and snorkel/”chimney” stenting. Surgery is a good option in patients of low or intermediate surgical risk and should remain a consideration during Heart Team discussions. Higher surgical risk patients currently have only two options—BASILICA or snorkel stenting. Snorkel stents are deployed between the TAVR frame and the aortic sinus or root. These stents are often under-expanded and risk being completely crushed which carries a high risk of acute or delayed coronary artery obstruction (13-15). Patients may remain on long-term dual antiplatelet therapy or anticoagulant which is undesirable in a population that tends to be elderly with multiple co-morbidities. Future coronary artery access will be challenging or impossible.
BASILICA can be performed with readily available, ‘off the shelf’ equipment, which negates the requirement for prolonged dual antiplatelet therapy, has an enlarging body of real world feasibility and safety data, and maintains coronary artery access in an orthotopic fashion for future de novo disease. Although the skill set may be unfamiliar to many operators, independent practice is expected following two to three proctored single leaflet cases.
Caveats
Several clinical situations require caution when considering BASILICA as they may risk coronary ostial obstruction despite successful leaflet laceration. Leaflet splay may be reduced during TAV-in-TAV procedures as the lacerated leaflet becomes pinned by the outer transcatheter heart valve frame (2). Moreover, BASILICA will not prevent coronary obstruction when stent posts of the degenerative transcatheter heart valve (THV) lie in front of a coronary ostium and are pushed outwards during delivery of the second valve (5). Eccentric coronary ostia may obstruct despite successful midline BASILICA laceration. Uncertainty remains as to the degree of leaflet splay experienced in vivo in patients with severely calcified and degenerative valve leaflets.
Future directions
Purpose-built guidewires, catheters and adjunctive tools are required to further simplify BASILICA techniques. Pachyderm-shaped guiding catheters (PAL1-3, PJR4) have already reduced time-to-leaflet traversal and with fewer crossing attempts per leaflet when compared to traditional techniques (16).
Mechanical leaflet laceration has recently been described with the ShortCut device (Pi-Cardia, Israel) but ability to cut through calcified leaflets is currently unknown. Excision of degenerative native or bioprosthetic valve leaflets is also an attractive concept, particularly in patients with eccentric coronary ostia, bulky leaflet calcification or who risk coronary obstruction following TAV-in-TAV with sinus effacement.
Acknowledgments
Funding: Supported by the Division of Intramural Research, National Heart, Lung and Blood Institute, National Institutes of Health, USA (Z01-HL006040).
Footnote
Conflicts of Interest: ABG—Proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular and he is a consultant for Transmural Systems. VCB—Consultant for Edwards Lifesciences and for Abbott Vascular, and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific. TR—Consultant and proctor for Edwards Lifesciences, Medtronic. On the Advisory Board for Medtronic. He has Equity Interest in Transmural Systems. JMK, TR, and RJL are co-inventors on patents, assigned to NIH, on catheter devices to lacerate valve leaflets. NHLBI has a collaborative research and development agreement with Edwards Lifesciences on transcatheter modification of the mitral valve. CGB has no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552-62. [Crossref] [PubMed]
- Khan JM, Bruce CG, Babaliaros VC, et al. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv 2020;13:787-9. [Crossref] [PubMed]
- Ribeiro HB, Rodés-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687-95. [Crossref] [PubMed]
- Forrestal BJ, Case BC, Yerasi C, et al. Risk of Coronary Obstruction and Feasibility of Coronary Access After Repeat Transcatheter Aortic Valve Replacement With the Self-Expanding Evolut Valve: A Computed Tomography Simulation Study. Circ Cardiovasc Interv 2020;13:e009496 [Crossref] [PubMed]
- Rogers T, Greenspun BC, Weissman G, et al. Feasibility of Coronary Access and Aortic Valve Reintervention in Low-Risk TAVR Patients. JACC Cardiovasc Interv 2020;13:726-35. [Crossref] [PubMed]
- Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc Interv 2018;11:677-89. [Crossref] [PubMed]
- Lederman RJ, Babaliaros VC, Rogers T, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv 2019;12:1197-216. [Crossref] [PubMed]
- Khan JM, Babaliaros VC, Greenbaum AB, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: Results From the Multicenter International BASILICA Registry. JACC Cardiovasc Interv 2021;14:941-8. [Crossref] [PubMed]
- Greenbaum AB, Kamioka N, Vavalle JP, et al. Balloon-Assisted BASILICA to Facilitate Redo TAVR. JACC Cardiovasc Interv 2021;14:578-80. [Crossref] [PubMed]
- Khan JM, Rogers T, Greenbaum AB, et al. Transcatheter Electrosurgery: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:1455-70. [Crossref] [PubMed]
- Khan JM, Greenbaum AB, Babaliaros VC, et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv 2019;12:1240-52. [Crossref] [PubMed]
- Khan JM, Greenbaum AB, Babaliaros VC, et al. BASILICA Trial: One-Year Outcomes of Transcatheter Electrosurgical Leaflet Laceration to Prevent TAVR Coronary Obstruction. Circ Cardiovasc Interv 2021;14:e010238 [Crossref] [PubMed]
- Jabbour RJ, Tanaka A, Finkelstein A, et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2018;71:1513-24. [Crossref] [PubMed]
- Mercanti F, Rosseel L, Neylon A, et al. Chimney Stenting for Coronary Occlusion During TAVR: Insights From the Chimney Registry. JACC Cardiovasc Interv 2020;13:751-61. [Crossref] [PubMed]
- Pighi M, Lunardi M, Pesarini G, et al. Intravascular ultrasound assessment of coronary ostia following valve-in-valve transcatheter aortic valve implantation. EuroIntervention 2021;16:1148-51. [Crossref] [PubMed]
- Lisko JC, Babaliaros VC, Lederman RJ, et al. Pachyderm-Shape Guiding Catheters to Simplify BASILICA Leaflet Traversal. Cardiovasc Revasc Med 2019;20:782-5. [Crossref] [PubMed]






