Sutureless repair techniques for post-infarction left ventricular free wall rupture
Introduction
Left ventricular free wall rupture (LVFWR) remains to be one of the most life-threatening complications of acute myocardial infarction (AMI). LVFWR occurs in up to 2% of patients with AMI (1). First myocardial infarction, anterior AMI, female sex, hypertension, and age >70 years have been reported to be significant risk factors for LVFWR (2). Conversely, primary percutaneous coronary intervention is a significant protective factor against LVFWR (2). Although the incidence of LVFWR has decreased in association with the widespread adoption of reperfusion therapy, the mortality rate remains as high as 50% (2). LVFWR still accounts for 24–61% of in-hospital disease-related mortality and requires prompt diagnosis and treatment (3).
Clinical presentation varies from cardiogenic shock or cardiac arrest with pericardial effusion to stable hemodynamics. Although conservative treatment for LVFWR has been reported (4), prompt surgical repair is the treatment of choice. However, the tissues at the rupture site are usually very vulnerable due to AMI, and therefore, careful surgical repair is required. To date, various surgical treatment options have been proposed. However, the optimal therapeutic strategy remains controversial because of the relatively low incidence of post-infarction LVFWR. In this review, the primary focus will be on the technique and outcomes of surgical repair, specifically sutureless repair techniques, for post-infarction LVFWR.
Types of LVFWR
According to the intraoperative findings, LVFWR has been historically classified into two types: blow-out and oozing. The oozing type is more frequently observed, and occurs in almost 80% of cases (5). The blow-out type is defined as a macroscopic defect of the epicardium with free communication between the left ventricular cavity and the pericardial space (6). The oozing type is defined as absence of a macroscopic tear on the infarcted area, with blood oozing from the infarcted myocardium (6). In some cases, bleeding due to LVFWR ceases spontaneously and only a hematoma on the infarcted myocardium, but no active bleeding, can be observed intraoperatively. This type of LVFWR, which might be called a sealed rupture, has been classified as the oozing type in most studies (3,7). Not surprisingly, the blow-out type confers a higher probability of early mortality compared to the oozing type (3,8).
History and types of surgical procedures
The principles of surgical repair for LVFWR include relieving cardiac tamponade, closing the tear, and obtaining hemostasis while minimizing damage to the intact myocardium. Prevention of postoperative re-rupture or pseudoaneurysm formation is also important.
Although percutaneous intrapericardial fibrin glue injection therapy has been reported as an alternative treatment (9), surgical repair is the gold standard in the treatment of LVFWR. Several different surgical repairs for LVFWR have been proposed since the 1970s and have varied over time. In 1972, FitzGibbon et al. reported infarctectomy and closure of the myocardial defect under cardiopulmonary bypass (CPB) (10). Montegut also reported successful surgical repair using a direct suturing technique for LVWFR (11). Later, surgical repair using a patch was introduced. For better control of hemorrhage, Núñez et al. described a Teflon patch repair covering the ventricular tear and the surrounding infarcted myocardium (12). A Teflon patch was fixed to the normal myocardium using a continuous suture. In their series, four of seven patients survived. Thereafter, with the advent of glue, several investigators have attempted to fix the patch onto the epicardium using glue without sutures. Padró et al. reported 13 cases of patch repair with glue (13), where a Teflon patch was placed over the area and glued to the heart surface with surgical glue. CPB was not used, except in a patient with a posterior tear. The outcome was excellent, with zero mortality up to five years postoperatively. In 2005, Muto et al. reported a successful case of sutureless repair using a fibrin tissue-adhesive collagen fleece (14).
As mentioned, several different techniques are currently available for the treatment of post-infarction LVFWR. They are generally classified into two groups: sutured and sutureless repair (15). In a meta-analysis of 25 studies performed by Matteucci et al., the outcomes of 209 patients who underwent surgery for LVFWR were reviewed (5). Sutured repair was used in 56% of patients; in-hospital mortality after sutured repair was 13.8%, which was comparable to 14% after sutureless repair. A trend toward a higher rate of in-hospital re-rupture was observed in patients who underwent sutureless repair. However, in a meta-analysis involving 363 patients, which was reported later by the same group, sutureless repair was shown to reduce in-hospital mortality by approximately 40% compared to suture repair (8).
Sutureless repair techniques
The infarcted myocardium is usually fragile and is difficult to suture. Thus, sutureless repair using tissue adhesive materials has the advantage of reducing trauma to an already infarcted area, without need to excise the myocardium or pass stitches through friable tissues. Left ventricular geometry can also be preserved. Furthermore, sutureless repair can be performed without the use of CPB and thus prevents the release of cytokines associated with the use of CPB, although the correlation between the use of CPB and in-hospital mortality has not been demonstrated (8). Currently, a variety of adhesive materials and/or glue are used for sutureless repair. In which cases sutureless repair can be safely performed remains controversial, due to the limited number of cases.
Several types of patches can be used for sutureless repair: autologous pericardium, xenopericardium, synthetic materials, and an extracellular matrix. Sakaguchi et al. reported the clinical outcomes of sutureless repair in 32 consecutive patients with oozing type LVFWR. They used an autologous pericardial patch and biological glue, and the in-hospital mortality rate was 15.6% (16). Holubec et al. used an acellular xenogeneic extracellular matrix membrane, which has excellent pliability, although running sutures were also performed around the infarcted area in addition to fixation with glue (17). Recently, the use of a collagen sponge patch for sutureless repair has been widely reported (7,14,18). TachoComb and TachoSil (CSL Behring K.K., Tokyo, Japan) are ready-to-use sheets of fibrin tissue-adhesive collagen fleece and contain fibrinogen, thrombin, and aprotinin (Figure 1). Unlike TachoComb, TachoSil is free from bovine aprotinin and can be stored at room temperature. Compared to pericardial patches, the advantage of the collagen patch is that it is fast and has excellent pliablity.
Surgical technique of sutureless repair using TachoSil
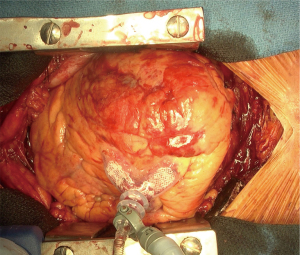
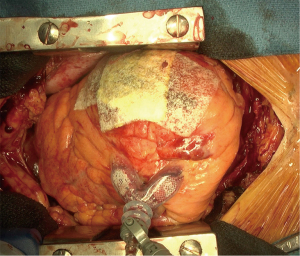
Median sternotomy and pericardiotomy were performed to relieve tamponade. After clots and blood were removed from the pericardial space, the rupture site was carefully identified (Figure 2). The heart was elevated either manually or using a Starfish heart positioner (Medtronic Inc., Minneapolis, MN, USA) if necessary. In general, CPB is not required. The tear or bleeding site on the epicardium and the extent of the hematoma on the heart were evaluated. Subsequently, TachoSil hemostatic collagen sponges were placed and manually pressed using gauze to the surface of the infarcted area (Figure 3). Usually, two or three sheets of TachoSil sponges are applied to cover the epicardial hematoma surrounding the tear. After hemostasis was confirmed, we additionally applied fibrin glue on the margin of the TachoSil patches to secure the adhesion of the patches on the epicardium, as we experienced a case of TachoSil patches that came off the myocardium early. A 77-year-old woman underwent sutureless repair using only TachoSil patches for LVFWR, and subsequently underwent reoperation for new-onset ventricular septal rupture four days postoperatively. In the second operation, we found that the TachoSil patches were completely detached from the myocardium. Although the TachoSil patch itself is a tissue-adhesive, we currently also apply glue around the TachoSil patches.
The outcomes of sutureless repair using TachoComb/TachoSil
Since a successful case was reported in 2005 (14), several investigators have reported the outcomes of sutureless repair using TachoComb/TachoSil, although the number of cases remain to be very small. Raffa et al. reported the outcomes of six consecutive patients who underwent sutureless repair using the TachoSil patch (18). Of the six patients, two had the blow-out type, and CPB was used in three patients with unstable hemodynamics. The in-hospital mortality rate was 17%. Postoperative re-rupture occurred in one of two patients with blow-out type LVFWR, which was successfully treated. We previously reported the outcomes of 35 patients who underwent sutureless repair using the TachoComb/TachoSil patch (7). The blow-out type was observed in two patients. None of the patients required CPB. The in-hospital mortality rate was 17%, and postoperative re-rupture occurred in six patients (17%). The rates of re-rupture were 12% in patients with the oozing type of LVFWR and 100% in those with the blow-out type.
Although sutureless repair is simple, fast, and reportedly associated with better early outcomes than suture repair (8), patients need to be carefully selected, especially those with a blow-out type of LVFWR. Several successful cases of sutureless repair for a blow-out type LVFWR have been reported (19,20); however, in the larger series, the incidence of postoperative re-rupture was very high in those with a blow-out type (7,18). Therefore, it is reasonable to think that sutureless repair is not recommended for the blow-out type and, if applied, postoperative close surveillance and early detection of re-rupture followed by surgical treatment are necessary. Furthermore, even in patients with an oozing type, intraoperative findings, such as oozing of blood from multiple areas of the epicardium, bulging of the infarcted myocardium in the systolic phase, severely edematous heart, and large hematoma in the epicardium, might be associated with postoperative re-rupture, and can help surgeons determine the appropriate surgical procedures (7).
Conclusions
Sutureless repair, especially using a collagen patch, can be a simple, fast, and reproducible treatment option for LVFWR in selected cases. Currently, sutureless repair is the best treatment option, especially for the oozing type; however, postoperative re-rupture remains an issue. Further large-scale studies are required to provide long-term data and evidence for the appropriate selection of treatment options.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nishiyama K, Okino S, Andou J, et al. Coronary angioplasty reduces free wall rupture and improves mortality and morbidity of acute myocardial infarction. J Invasive Cardiol 2004;16:554-8. [PubMed]
- Honda S, Asaumi Y, Yamane T, et al. Trends in the clinical and pathological characteristics of cardiac rupture in patients with acute myocardial infarction over 35 years. J Am Heart Assoc 2014;3:e000984. [Crossref] [PubMed]
- Formica F, Mariani S, Singh G, et al. Postinfarction left ventricular free wall rupture: a 17-year single-centre experience. Eur J Cardiothorac Surg 2018;53:150-6. [Crossref] [PubMed]
- Nasir A, Gouda M, Khan A, et al. Is it ever possible to treat left ventricular free wall rupture conservatively? Interact Cardiovasc Thorac Surg 2014;19:488-93. [Crossref] [PubMed]
- Matteucci M, Fina D, Jiritano F, et al. Sutured and sutureless repair of postinfarction left ventricular free-wall rupture: a systematic review. Eur J Cardiothorac Surg 2019;56:840-8. [Crossref] [PubMed]
- Haddadin S, Milano AD, Faggian G, et al. Surgical treatment of postinfarction left ventricular free wall rupture. J Card Surg 2009;24:624-31. [Crossref] [PubMed]
- Okamura H, Kimura N, Mieno M, et al. Sutureless repair for postinfarction left ventricular free wall rupture. J Thorac Cardiovasc Surg 2019;158:771-7. [Crossref] [PubMed]
- Matteucci M, Formica F, Kowalewski M, et al. Meta-analysis of surgical treatment for postinfarction left ventricular free-wall rupture. J Card Surg 2021;36:3326-33. [Crossref] [PubMed]
- Terashima M, Fujiwara S, Yaginuma GY, et al. Outcome of percutaneous intrapericardial fibrin-glue injection therapy for left ventricular free wall rupture secondary to acute myocardial infarction. Am J Cardiol 2008;101:419-21. [Crossref] [PubMed]
- FitzGibbon GM, Hooper GD, Heggtveit HA. Successful surgical treatment of postinfarction external cardiac rupture. J Thorac Cardiovasc Surg 1972;63:622-30. [Crossref] [PubMed]
- Montegut FJ Jr. Left ventricular rupture secondary to myocardial infarction. Report of survival with surgical repair. Ann Thorac Surg 1972;14:75-8. [Crossref] [PubMed]
- Núñez L, de la Llana R, López Sendón J, et al. Diagnosis and treatment of subacute free wall ventricular rupture after infarction. Ann Thorac Surg 1983;35:525-9. [Crossref] [PubMed]
- Padró JM, Mesa JM, Silvestre J, et al. Subacute cardiac rupture: repair with a sutureless technique. Ann Thorac Surg 1993;55:20-3. [Crossref] [PubMed]
- Muto A, Nishibe T, Kondo Y, et al. Sutureless repair with TachoComb sheets for oozing type postinfarction cardiac rupture. Ann Thorac Surg 2005;79:2143-5. [Crossref] [PubMed]
- Matteucci M, Fina D, Jiritano F, et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care 2019;8:379-87. [Crossref] [PubMed]
- Sakaguchi G, Komiya T, Tamura N, et al. Surgical treatment for postinfarction left ventricular free wall rupture. Ann Thorac Surg 2008;85:1344-6. [Crossref] [PubMed]
- Holubec T, Caliskan E, Bettex D, et al. Repair of post-infarction left ventricular free wall rupture using an extracellular matrix patch. Eur J Cardiothorac Surg 2015;48:800-3. [Crossref] [PubMed]
- Raffa GM, Tarelli G, Patrini D, et al. Sutureless repair for postinfarction cardiac rupture: a simple approach with a tissue-adhering patch. J Thorac Cardiovasc Surg 2013;145:598-9. [Crossref] [PubMed]
- Nishizaki K, Seki T, Fujii A, et al. Sutureless patch repair for small blowout rupture of the left ventricle after myocardial infarction. Jpn J Thorac Cardiovasc Surg 2004;52:268-71. [Crossref] [PubMed]
- Lachapelle K, deVarennes B, Ergina PL, et al. Sutureless patch technique for postinfarction left ventricular rupture. Ann Thorac Surg 2002;74:96-101. [Crossref] [PubMed]