Therapeutic alternatives in chronic thromboembolic pulmonary hypertension: from pulmonary endarterectomy to balloon pulmonary angioplasty to medical therapy. State of the art from a multidisciplinary team
Introduction
As the name reflects, chronic thromboembolic pulmonary hypertension (CTEPH) is a type of pulmonary hypertension (PH) that is long lasting and evolving, secondary to peripheral venous clot embolization and subsequent intravascular thrombosis. It is classified as World Health Organization (WHO) group 4 PH. CTEPH is formally defined as (I) mean pulmonary artery pressure (mPAP) of 25 mmHg or higher and a pulmonary capillary wedge pressure of 15 mmHg or lower, (II) present after at least three months of effective anticoagulant therapy and (III) confirmation of organized thrombi in the pulmonary arteries by pulmonary angiography (1-3). However, two terms are currently used to describe symptomatic patients with chronic thromboembolic occlusions of pulmonary arteries according to the presence or absence of PH at rest: chronic thromboembolic pulmonary hypertension (CTEPH) and chronic thromboembolic disease (CTED), respectively (4). A change in the definition of PH to decrease the mPAP threshold from 25 to 21 mmHg and pulmonary vascular resistance (PVR) from three to two Wood units has been proposed (5).
In both cases, the natural history of the disease is very heterogeneous, as is the disease onset, anatomy and degree of hypertension. What is known is that the long-term prognosis is very poor, with progression to cardiac and pulmonary involvement, and if left untreated, can lead to compensatory mechanisms such as vascular remodeling. This can further exacerbate the degree of PH and right ventricular dysfunction, making the disease irreversible or less responsive to any proposed treatment (6,7). It is reasonable that early diagnosis is crucial to improve disease prognosis and the results of different therapies. Unfortunately, early diagnosis is not always feasible. Theoretically, patients with CTEPH should have had a previous episode of acute pulmonary embolism (PE), but it is estimated that fewer than 40% of patients have had an acute episode, which makes the differential diagnosis more difficult (8,9). This is due to the few or even lack of symptoms during the acute process, when thromboembolic fragments are of small caliber, in limited number and tend to obstruct distal arterial branches. Another reason that could explain the lack of a previous acute PE in some cases is that an “in situ” thrombosis of distal vessels can occur, particularly in patients who are prothrombotic. Moreover, the incidence of CTEPH after an acute PE is still not well identified, ranging from 0.6% to 3.2% (10). Regardless of the initial phenomenon, the fresh thrombi adhere to the pulmonary vascular wall and undergo fibrosis, giving rise to a plaster-like material that reduces or obstructs the lumen of the vessel (Figure 1). Furthermore, the pulmonary vascular bed not involved in the thromboembolic process tends to modify its vascular tone to regulate the overflow from the occluded areas. The protracted vasoconstriction leads to changes in the microarchitecture of the pulmonary vessel walls, similar to that of pulmonary venules in the context of post-capillary PH secondary to left ventricular heart disease in veno-occlusive disease. Vascular remodeling is also potentiated by a combination of defective angiogenesis, impaired fibrinolysis and endothelial dysfunction. In addition, substantial pre- and post-capillary broncho-pulmonary anastomoses lead to the transmission of systemic blood pressure to the pulmonary circulation (11,12). This complex pathophysiology explains why the statement “pulmonary endarterectomy (PEA) is curative” has been debunked. It is true that the ideal and curative treatment would be PEA, but its success depends on the degree of PH and how distal or accessible the disease is. As with any other major open heart operation, outcome also depends on the patient’s conditions and the center experience. This is the reason why not every patient with CTEPH has all the ideal characteristics to be a good candidate for PEA. In the literature, fewer than 60% of patients with a confirmed diagnosis of CTEPH have undergone PEA (13). Thus, the high percentage of patients considered inoperable has made the development of alternative therapies necessary to improve both the clinical conditions and the survival of patients suffering from this serious disease.
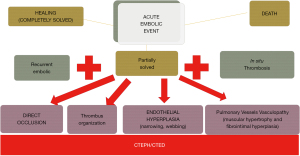
In the present keynote, we attempt to give a useful and introductory guide to the other extraordinary works published in this issue that discuss the main therapeutic options and its roles in the context of CTEPH: medical therapy, surgical therapy and interventional therapy. The synergistic work between the different specialists in the treating team is necessary to offer the treatment with the best risk-to-benefit ratio.
Pulmonary endarterectomy
Guidelines from both the American and European Society of Cardiology endorse PEA [also known as pulmonary thromboendarterectomy (PTE)] as a Class I recommendation with Level C evidence in patients affected by proximal and accessible CTEPH (14,15). Prior to the team discussion involving a PH cardiologist, radiologist, interventionist, cardiac surgeon and anesthesiologist, all patients must undergo echocardiography, ventilation-perfusion (VQ) scanning, right heart catheterization (RHC), CT pulmonary angiography and coronary angiography to meet the diagnostic criteria of CTEPH and to categorize the patient. At our center, every case of CTEPH is discussed and after a comprehensive evaluation of all data and images, risks and benefits are weighed alongside an extensive discussion with the patient, and a final therapeutic decision is made.
The reasons for not suggesting or contraindicating PEA as a first-line treatment are the associated high-risk comorbidities or surgically inaccessibility of certain thromboembolic lesions, even though one of the most important surgical advances has been the distal access of endarterectomy. Distal lesions in a context of associated pathologies and perhaps older age, used to be a strong deterrent for surgery. Thanks to increasing diagnostic and surgical experience, PEA can now be successfully performed in some patients with distal chronic thromboembolism (16,17).
Experienced centers should include expert cardiothoracic surgeons familiar with complex procedures requiring deep hypothermic circulatory arrest (DHCA), a case volume of >50 procedures per annum, ≥5 years of experience, an in-hospital mortality of <5%, the ability to perform distal endarterectomy and offer all three modalities of treatment. Also, PEA centers should be able to provide extracorporeal membrane oxygenation (ECMO) (18-20).
Although the European Respiratory Society task force did not delve into the technical aspects of the surgical operation, there is consensus that the procedure is performed through a median sternotomy on extracorporeal circulation. The safe completion of endarterectomy, especially for the removal of the most distal fibrotic thrombus, requires a bloodless field, which is obtained only with circulatory arrest (Figure 2). Organ protection during circulatory arrest is obtained by lowering the body temperature. As a result, experts advise that PEA should be performed under DHCA at 20 degrees limited to 20-minute intervals. One period is enough for the accomplishment on each side. Identification of the correct plane is crucial to prevent perforation of the pulmonary artery (Figure 3) (21-23). The International CTEPH Registry reported a <5% incidence of postoperative mortality from seventeen centers, although a larger patient series from a single center reported <2.2% mortality rate (24,25).
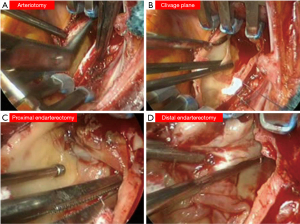
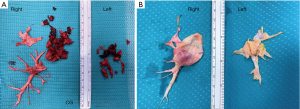
PEA is the most effective treatment for CTEPH as recently reported in a meta-analysis (26). The longest complete follow up from the UK National Series demonstrated a 72% survival rate at ten years, with almost 50% of deaths not related to CTEPH. From a functional point of view, a group from Pavia, Italy reported the longest follow up for functional class, with 74% of patients in class NYHA II at four years after surgery (27).
There is no current definition of success after PEA surgery, and results of PEA are determined by patient-specific characteristics. Although patients should expect to survive the operation without cognitive dysfunction or major morbidity, residual PH following PEA is not rare. This is one of the main indications for ECMO in the early postoperative phase, particularly when severe. In the long term, true recurrence is rare and patients are mostly affected by residual PH. As residual PH predicts CTEPH-related deaths, medical therapy as well as balloon pulmonary angioplasty (BPA) in this setting have an important role (28,29).
Medical treatment: pulmonary vasodilators
Basic therapies for CTEPH include lifelong anticoagulation, diuretics and oxygen in hypoxic patients. Regarding anticoagulation, vitamin K inhibitors (VKI) are the mainstay. Novel oral anti-coagulants (NOAC) are increasingly used, but more evidence is needed regarding its efficacy, safety and interactions.
Although PEA remains the treatment of choice for most patients with CTEPH, around 40% of patients in the International CTEPH Registry were considered inoperable due to concerns for inaccessible vascular obstruction, mPAP out of proportion to morphological lesions and significant prohibitive comorbidities (30). Inoperable patients and patients with residual persistent or recurrent PH after PEA should be treated with PH-specific medications to improve symptoms and hemodynamics (31).
In 2014, a soluble guanylate- cyclase stimulator (sGC), riociguat, which targets the nitric oxide pathway with a pulmonary vasodilatory effect, received approval for insurance reimbursement in the context of inoperable or persistent/recurrent CTEPH. This was based on the findings of a multi-center randomized controlled trial (RCT), CHEST-1 (28). Riociguat is an effective pulmonary vasodilator and is associated with a low risk of serious adverse events. It has been reported that sequential treatment with riociguat and BPA results in significant improvements in terms of mPAP and PVR among patients with inoperable CTEPH. Currently, Riociguat is also being tested in RCTs for its efficacy and safety as bridging therapy for patients scheduled to undergo PEA.
Currently, sildenafil, a phosphodiesterase type 5 inhibitor (PDE5i) is not approved for use in CTEPH, as studies regarding its efficacy have insufficient power (32).
Recently, the MERIT-1 trial showed that macitentan [an endothelin receptor antagonist (ERA)] improved PVR (P=0.041), six-minute walk distance (P=0.033) and n-terminal pro-brain natriuretic peptide (P=0.040) in patients with inoperable CTEPH, providing evidence of combination drug therapy (33). The use of medical therapy as a bridge to PEA is still controversial, even though there is consensus about the microvascular component of the PH. Medical therapy before surgery is felt to delay referral without demonstrable clinical benefit. In the international registry, pre-treatment, even independently, predicted worse outcome (hazard ratio 2.62; P=0.0072) (24). However pulmonary vasodilators while attending the operation is common in the clinical practice, and its efficacy is currently being studied in the ongoing riociguat RCTs.
It is suggested that in case of residual PH after PEA, initiation of medical therapy with pulmonary vasodilators, specifically with riociguat, might be consider when mPAP rises up to 30 mmHg (26). The 2018 World Symposium on Pulmonary Hypertension (WSPH) treatment algorithm recommended medical therapy and consideration of BPA or redo-PEA in patients with persistent symptomatic PH following PEA (15).
Interventional approach: balloon pulmonary angioplasty
BPA has had a recent renaissance thanks to technical refinements from Japan (34-36). BPA was first reported in 2001 but was not included in the therapeutic armamentarium of CTEPH due to high, potentially lethal, complications rates. BPA has been demonstrated to improve hemodynamic parameters. It also yields other positive effects, like cardiac function, quality of life and exercise capacity, not only in CTEPH patients, but also in chronic thromboembolic disease (CTED) cases (37). BPA is indicated in cases of inoperable CTEPH, after a multidisciplinary discussion and weighing of risks and benefits of PEA. The European Society of Cardiology and the European Respiratory Society have a Class I recommendation with Level IIb for the use of BPA in the guidelines for the treatment of PH (14,38).
Several articles in the current issue are dedicated to the technical aspects of BPA. The main message is that BPA requires multiple sessions and dilatations in order to obtain optimal results safely. Reperfusion edema is considered one of the major and frequent complications, even as the rate of complications has decreased significantly since the first reports in 2001. The incidence varies in each report, depending on how each complication is defined and counted (37,39).
BPA may also have a role in patients with residual PH after PEA. Cases series report an improvement in hemodynamics and exercise capacity following BPA, with the same rate of complications as non-operable CTEPH. Most of these patients were treated with PH-targeting drugs prior to BPA (29,40,41).
There is not enough information to make a statement about the role of BPA as bridging therapy prior to PEA and this area warrants more research (42).
Multidisciplinary therapuetic approach
CTEPH patients used to have a wide phenotypical heterogeneity due to the pathophysiology and natural course of the disease. They might have mixed lesions with proximal, segmental occlusions, stenosis and webs, etc., with different degrees of PH. Thus, the current treatment paradigm includes a multimodal management approach, which involves employing a combination of PEA for proximal lesions, BPA for very distal lesions and targeted pharmacological therapy for microvasculopathy. Due to the lack of guidance regarding multimodal therapeutic management of CTEPH, patient selection and treatment is individualized and customized, based on the expertise of the multidisciplinary team.
Recent cohorts have, however, demonstrated that in general, revascularization of targeted vessels, including with PEA and BPA, have similar long-term results in terms of survival and CTEPH-related deaths compared with medical therapy alone (43).
Conclusions
CTEPH is a rare, or at least under-recognized type of PH resulting from thrombotic occlusion (embolic or not embolic) of the pulmonary arteries at different levels. As it is not easy to recognize, it is the duty of PH experts to familiarize the medical community with the disease as soon as possible in order allow for early referral to a PH expert center.
Expert centers must be constituted by a multidisciplinary team, offering a complete diagnostic pathway and the best customized treatment. Multimodal treatment must inevitably include expert radiologists and PH cardiologist in order to make the correct diagnosis, medically treat inoperable patients and those with residual PH after PEA, and categorize the disease in terms of anatomy, degree of PH etc. Similarly, an expert team composed of surgeons, anesthesiologists and interventionist must be able to offer the best results of PEA or BPA after carefully weighing the risks and the benefits. The lack of all these components would inevitably result in the failure of the diagnostic and therapeutic process.
The aim of the present issue is to practically summarize CTEPH to every physician interested in the subject and highlight the priceless contribution of the outstanding authors who developed all aspects related to CTEPH, pulmonary endarterectomy and all therapeutic alternatives.
Acknowledgments
I’d like to thank both Professor Marco Di Eusanio and Professor Jose Luis Pomar for them costant support and unvaluable friendship.
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jenkins D, Mayer E, Screaton N, et al. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev 2012;21:32-9. [Crossref] [PubMed]
- Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81:151-8. [Crossref] [PubMed]
- Coulden R. State-of-the-art imaging techniques in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc 2006;3:577-83. [Crossref] [PubMed]
- Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021;57:2002828. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62:D92-9. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Lang IM, Pesavento R, Bonderman D, et al. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013;41:462-8. [Crossref] [PubMed]
- Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014;130:508-18. [Crossref] [PubMed]
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64. [Crossref] [PubMed]
- Boulate D, Perros F, Dorfmuller P, et al. Pulmonary microvascular lesions regress in reperfused chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2015;34:457-67. [Crossref] [PubMed]
- Dorfmüller P, Günther S, Ghigna MR, et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J 2014;44:1275-88. [Crossref] [PubMed]
- Miniati M, Monti S, Bottai M, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006;85:253-62. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1801915. [Crossref] [PubMed]
- Madani M, Mayer E, Fadel E, et al. Pulmonary Endarterectomy. Patient Selection, Technical Challenges, and Outcomes. Ann Am Thorac Soc 2016;13:S240-7. [Crossref] [PubMed]
- D'Armini AM, Morsolini M, Mattiucci G, et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2014;148:1005-11; 1012.e1-2; discussion 1011-2.
- Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141:702-10. [Crossref] [PubMed]
- Boulate D, Mercier O, Mussot S, et al. Extracorporeal Life Support After Pulmonary Endarterectomy as a Bridge to Recovery or Transplantation: Lessons From 31 Consecutive Patients. Ann Thorac Surg 2016;102:260-8. [Crossref] [PubMed]
- Madani M, Ogo T, Simonneau G. The changing landscape of chronic thromboembolic pulmonary hypertension management. Eur Respir Rev 2017;26:170105. [Crossref] [PubMed]
- Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160111. [Crossref] [PubMed]
- Jamieson SW, Kapelanski DP. Pulmonary endarterectomy. Curr Probl Surg 2000;37:165-252. [Crossref] [PubMed]
- Madani MM, Jamieson SW. Pulmonary endarterectomy for chronic thromboembolic disease. Oper Tech Thorac Cardiovasc Surg 2006;11:264-74. [Crossref]
- Delcroix M, Lang I, Pepke-Zaba J, et al. Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension: Results From an International Prospective Registry. Circulation 2016;133:859-71. [Crossref] [PubMed]
- Hsieh WC, Jansa P, Huang WC, et al. Residual pulmonary hypertension after pulmonary endarterectomy: A meta-analysis. J Thorac Cardiovasc Surg 2018;156:1275-87. [Crossref] [PubMed]
- Cannon JE, Su L, Kiely DG, et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016;133:1761-71. [Crossref] [PubMed]
- Corsico AG, D'Armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med 2008;178:419-24. [Crossref] [PubMed]
- Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319-29. [Crossref] [PubMed]
- Shimura N, Kataoka M, Inami T, et al. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2015;183:138-42. [Crossref] [PubMed]
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011;124:1973-81. [Crossref] [PubMed]
- Pepke-Zaba J, Ghofrani HA, Hoeper MM. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160107. [Crossref] [PubMed]
- Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008;134:229-36. [Crossref] [PubMed]
- Ghofrani HA, Simonneau G, D'Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 2017;5:785-94. [Crossref] [PubMed]
- Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748-55. [Crossref] [PubMed]
- Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756-62. [Crossref] [PubMed]
- Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012;76:485-8. [Crossref] [PubMed]
- Ikeda N. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther 2020;35:130-41. [Crossref] [PubMed]
- Fukuda K, Date H, Doi S, et al. Guidelines for the Treatment of Pulmonary Hypertension (JCS 2017/JPCPHS 2017). Circ J 2019;83:842-945. [Crossref] [PubMed]
- Khan MS, Amin E, Memon MM, et al. Meta-analysis of use of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2019;291:134-9. [Crossref] [PubMed]
- Yanaka K, Nakayama K, Shinke T, et al. Sequential Hybrid Therapy With Pulmonary Endarterectomy and Additional Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J Am Heart Assoc 2018;7:008838. [Crossref] [PubMed]
- Araszkiewicz A, Darocha S, Pietrasik A, et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2019;278:232-7. [Crossref] [PubMed]
- Kawashima T, Yoshitake A, Kawakami T, et al. Two-stage Treatment Using Balloon Pulmonary Angioplasty and Pulmonary Endarterectomy in a Patient with Chronic Thromboembolic Pulmonary Hypertension. Ann Vasc Surg 2018;49:315.e5-7. [Crossref] [PubMed]
- Hu S, Tan JS, Liu S, et al. The long-term survival in patients with chronic thromboembolic pulmonary hypertension: experience from a single center in China. J Thromb Thrombolysis 2021; [Crossref] [PubMed]






