Pulmonary endarterectomy in severe chronic thromboembolic pulmonary hypertension: the Toronto experience
Introduction
Pulmonary endarterectomy (PEA) is the treatment of choice for chronic thromboembolic pulmonary hypertension (CTEPH). The surgery can be done with an operative mortality of less than 5%, a rate of blood transfusion of less than 50% and a hospital stay of less than two weeks on average (1). The results are excellent in the long-term with five-year survival greater than 80% (2). The majority of patients will not require any additional therapy other than life-long anticoagulation (3).
Preoperative pulmonary vascular resistance (PVR) is one of the most important predictors of postoperative mortality after PEA. According to the International CTEPH Registry, PVR greater than 1,200 dynes.s.cm–5 at diagnosis is associated with a significant rise in hospital mortality compared to PVR of less than 800 dynes.s.cm–5, with mortality rate of 10.6% compared to 2.8%, respectively (4). Other groups have also observed that preoperative PVR greater than 1,000 dynes.s.cm–5 was associated with an operative mortality greater than 10% (5-9). More recently, the group from University of California, San Diego (UCSD) has shown that with increasing experience, the hospital mortality can decrease to less than 5% in patients with preoperative PVR >1,000 dynes.s.cm–5 (5). In our experience in Toronto, the option of post-operative extra-corporeal membrane oxygenation (ECMO) has also provided increased safety in operating on patients with severely increased PVR, particularly in the context of decompensated right heart failure (RHF) and segmental disease. This option has seen hospital mortality decrease from 13.2% to 1.7% (10).
The long-term outcome after PEA in the context of severely increased preoperative PVR is not well defined. Some studies suggest that preoperative PVR is an independent predictor of mortality up to three years after PEA, although others do not (3,11). It is also increasingly recognized that some patients will benefit from targeted pulmonary hypertension (PH) medical therapy or balloon pulmonary angioplasty (BPA) after PEA, but the impact of preoperative PVR on the need for additional therapy after PEA has not been analyzed systematically (3). The primary objectives of this study were therefore focused on the early and long-term results after PEA in patients with PVR >1,000 dynes.s.cm–5 at the time of diagnosis compared to patients with lower PVR.
Methods
After approval by the Toronto General Hospital Institutional Research Ethics Board for waived consent (19-5181), we performed a retrospective analysis of a prospectively collected consecutive cohort of adult patients undergoing PEA at the Toronto General Hospital between August 2005 and March 2020. Patients undergoing PEA for sarcoma and vascular malignancies were excluded.
PEA was performed on cardiopulmonary bypass (CPB) with deep hypothermic circulatory arrest (DHCA) at 20 °C based on the bladder temperature probe, with at least one circulatory arrest for each side (3). Briefly, after median sternotomy, CPB was initiated using a 22 Fr aortic cannula and two single stage 32 and 36 Fr venous cannula advanced into the superior and inferior vena cava, respectively. Once at 24 °C, the aorta was cross-clamped and 1 liter of cold high potassium cardioplegia was given. Cold cardioplegia was repeated every 20 to 30 minutes. The right pulmonary artery (PA) was opened from behind the aorta to the right upper pulmonary vein and the plane of endarterectomy elevated in each segment. Circulatory arrest was then performed at 20 °C. Each circulatory arrest was limited to 20 minutes. After completion of the right side, the PEA was performed on the left side in a similar manner using a curved incision on the anterior aspect of the left PA.
Central veno-arterial (VA) ECMO has been used since 2014 in the presence of difficulty weaning off CPB after PEA based on hemodynamic parameters and gas exchange (10). Peripheral VA ECMO was generally used postoperatively in the presence of worsening hemodynamic instability due to RHF. Veno-venous (VV) ECMO was used postoperatively in the presence of isolated respiratory failure due to reperfusion pulmonary edema or severe intrapulmonary shunting leading to severe hypoxemia.
The PEA specimen was characterized at the time of surgery according to the Jamieson classification (12). Type 1 was characterized by fresh thrombus in the main-lobar pulmonary arteries, type 2 by intimal thickening and fibrosis with or without organized thrombus proximal to segmental arteries, type 3 by fibrosis, intimal webbing and thickening with or without organized thrombus within the segmental arteries only, and type 4 by the absence of visible thromboembolic disease. Type 4 disease was limited to five patients. These patients were mostly identified early in our experience and grouped with type 3 as the absence of visible thromboembolic disease may have been related to the learning curve in the context of segmental disease.
Severe CTEPH was defined by PVR greater than 1,000 dynes.s.cm–5 at the time of diagnosis. A comparative study was then performed between patients with pre-operative PVR >1,000 dynes.s.cm–5 and those with PVR <1,000 dynes.s.cm–5. Patients were occasionally treated preoperatively with targeted PH medical therapy by the local PH team. If initiated, targeted PH medical therapy was stopped at the time of PEA. After PEA, patients recovered in Toronto until their discharge from hospital. Patients were then referred back to their home PH centers across Canada for further follow-up. Decision to initiate targeted PH medical therapy after PEA was made by the referring PH centers. All patients were followed in the long-term by the referring PH centers and the date of death or last follow-up was recorded.
Demographics and other results were reported as mean ± standard deviation (SD), or median and interquartile range (IQR). Categorical variables were compared by chi-squared analysis and continuous variables by Student’s t-test. Survival was determined by using the Kaplan-Meier method. The log-rank test was used to determine survival differences. Threshold for statistical significance was a P value less or equal to 0.05. Statistical analysis was performed using GraphPad Prism, Version 8, https://www.graphpad.com/scientific-software/prism/.
Results
Demographic data
A total of 401 patients underwent PEA during the study period. One hundred had PVR >1,000 dynes.s.cm–5 and 301 had PVR <1,000 dynes.s.cm–5. Patients’ age and sex were similar between both groups (Table 1). The New York Heart Association (NYHA) functional class, 6-minute walk distance and brain natriuretic peptide (BNP) were worse in patients with PVR >1,000 dynes.s.cm–5 (Table 1). A greater proportion of patients with PVR >1,000 dynes.s.cm–5 was on home oxygen. The proportion of patients with PVR >1,000 dynes.s.cm–5 was similar between patients living in Ontario and those referred from other Canadian provinces (32% vs. 33%, respectively; P=0.9).
Table 1
| Characteristics | PVR >1,000 dynes.s.cm–5 (n=100) | PVR <1,000 dynes.s.cm–5 (n=301) | P value |
|---|---|---|---|
| Age (years) | 58±15 | 58±15 | 0.99 |
| Sex | |||
| Male | 44 (44%) | 154 (51%) | 0.21 |
| Female | 56 (56%) | 147 (49%) | |
| NYHA functional class | <0.001 | ||
| I−II | 7 (7%) | 87 (29%) | |
| III−IV | 93 (93%) | 214 (71%) | |
| Home oxygen | 0.02 | ||
| Yes | 29 (29%) | 53 (18%) | |
| No | 71 (71%) | 248 (82%) | |
| 6-minute walk distance (m) | 315±159 | 387±138 | <0.001 |
| Brain natriuretic peptide (pg/mL) | 597±571 | 182±295 | <0.001 |
| Right heart catheterization | |||
| RAP | 12±6 | 10±6 | 0.004 |
| PAP systolic | 90±10 | 66±21 | <0.001 |
| PAP diastolic | 34±9 | 24±8 | <0.001 |
| PAP mean | 55±10 | 40±12 | <0.001 |
| PCWP | 11±5 | 12±5 | 0.08 |
| Cardiac index | 1.6±0.4 | 2.3±0.6 | <0.001 |
| PVR | 1374±378 | 544±248 | <0.001 |
| TPR | 1575±438 | 724±278 | <0.001 |
| Targeted PH medical therapy | <0.001 | ||
| Yes | 38 (38%) | 55 (18%) | |
| No | 62 (62%) | 246 (82%) | |
| Hospital admission for RHF | 26 (26%) | 14 (5%) | <0.001 |
NYHA, New York Heart Association; RAP, right atrial pressure; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; TPR, total pulmonary resistance; PH, pulmonary hypertension; RHF, right heart failure.
Right heart catheterization demonstrated worse parameters in patients with PVR >1,000 dynes.s.cm–5, with the exception of the pulmonary capillary wedge pressures (Table 1). Targeted PH medical therapy was initiated preoperatively by the referring PH centers in 38% of the patients with PVR >1,000 dynes.s.cm–5 compared to 18% in patients with PVR <1,000 dynes.s.cm–5. Hospital admission for advanced RHF was documented in 26% of the patients with PVR >1,000 dynes.s.cm–5 compared to 4.7% in patients with PVR <1,000 dynes.s.cm–5 (P<0.001). Inotropic support in the intensive care unit (ICU) was required in 7 patients (7%) with PVR >1,000 dynes.s.cm–5 compared to 1 patient (0.3%) with PVR <1,000 dynes.s.cm–5 (P<0.001).
Perioperative outcomes
The duration of CPB was similar between patients with PVR greater and lower than 1,000 dynes.s.cm–5. The duration of circulatory arrest was, however, longer in patients with PVR >1,000 dynes.s.cm–5 (Table 2). A total of twenty-one patients underwent combined procedures with coronary artery bypass graft (n=20) or mitral valve replacement (n=1), with a similar proportion amongst both groups. An additional six patients underwent resection of an intracardiac mass, atrial septal defect (ASD) closure or sinus venosus ASD closure.
Table 2
| Characteristics | PVR >1,000 dynes.s.cm–5 (n=100) | PVR <1,000 dynes.s.cm–5 (n=301) | P value |
|---|---|---|---|
| Duration | |||
| CBP (min) | 252±32 | 246±41 | 0.18 |
| Aortic cross clamp (min) | 138±26 | 130±29 | 0.01 |
| Circulatory arrest (min) | 46±15 | 40±13 | 0.001 |
| Combined procedure | |||
| CABG, valve replacement | 8 (8%) | 13 (4%) | 0.15 |
| Jamieson classification | 0.24 | ||
| Type 1–2 | 69 (69%) | 190 (63%) | |
| Type 3–4 | 31 (31%) | 111 (37%) | |
| Improvement in TPR | 1,088±473 | 373±273 | <0.001 |
| Duration of intubation (days) | |||
| Mean ± SD | 5.8±8.3 | 3.6±5.5 | 0.003 |
| Median [IQR] | 3 [2–6] | 2 [1–4] | |
| Duration of ICU (days) | |||
| Mean ± SD | 9.2±10.6 | 5.8±6.3 | 0.001 |
| Median [IQR] | 5 [4–9] | 4 [2–6] | |
| Duration of hospital stay (days) | |||
| Mean ± SD | 21.9±17.9 | 17.1±18.3 | 0.02 |
| Median [IQR] | 15 [11–27] | 12 [9–17] | |
| Post-operative ECMO | 12 (12%) | 8 (3%) | 0.002 |
| 30-day mortality | 4 (4%) | 6 (2%) | 0.26 |
CPB, cardiopulmonary bypass; CABG, coronary artery bypass grafting; TPR, total pulmonary resistance; SD, standard deviation; IQR, interquartile range; ECMO, extracorporeal membrane oxygenation.
The type of surgical specimen was similar between both groups, with disease localized in the segmental PA branches and more distally (Type 3 disease) in 31% of the patients with PVR >1,000 dynes.cm–5 compared to 37% in patients with PVR <1,000 dynes.s.cm–5 (Table 2). Among patients with PVR >1,000 dynes.s.cm–5, postoperative improvement was similar between patients with Jamieson type 1–2 disease and type 3 disease (Figure 1). The PVR continued to improve beyond the immediate arrival in the ICU after surgery in both groups (Figure 1).

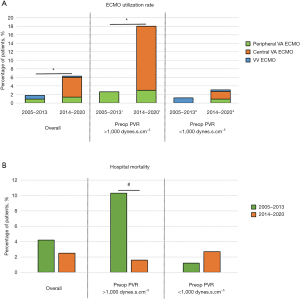
The duration of intubation, ICU stay, and hospital stay was longer in patients with PVR >1,000 dynes.s.cm–5 (Table 2). The main reason for delayed extubation was pulmonary edema/pneumonia. The median duration of intubation was three days in patients with PVR >1,000 dynes.s.cm–5 and two days in patients with PVR <1,000 dynes.s.cm–5 (Table 2). The overall 30-day mortality was 2.5%. The overall utilization rate of post-operative ECMO was 5%. After 2014, the ECMO utilization rate was higher in patients with PVR >1,000 dynes.s.cm–5 compared to those with PVR <1,000 dynes.s.cm–5 (18% vs. 3.1%, respectively; P<0.001). The use of central VA ECMO increased after 2014 in patients with PVR >1,000 dynes.s.cm–5 (Figure 2A). Central VA ECMO was predominantly used in patients with advanced RHF requiring hospital admission in the context of segmental disease (10). The increased use of central VA ECMO since 2014 was associated with a drop in hospital mortality in patients with PVR >1,000 dynes.s.cm–5 from 10.3% in 2005–2013 to 1.6% in 2014–2020 (Figure 2B). The hospital mortality (1.2% in 2005–2013 vs. 2.7% in 2014–2020, P=0.4) and ECMO utilization rate (1.2% in 2005–2013 vs. 3.2% in 2014–2020, P=0.4) did not significantly change in patients with PVR <1,000 dynes.s.cm–5 during the same time period.
Outcomes at six-month, one-year, and two-year
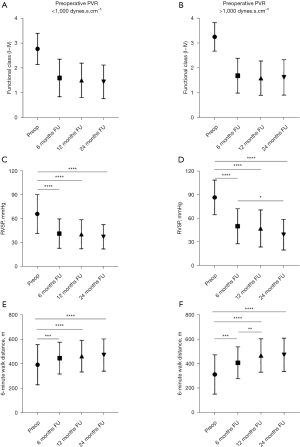
The improvement in NYHA functional class at six-month, one-year and two-year follow-up after PEA was very similar despite the preoperative PVR being greater or lower than 1,000 dynes.s.cm–5 (Figure 3). The right ventricular systolic pressure (RVSP) on echocardiogram and the 6-minute walk distance were also very similar between the two groups at six-month, one-year and two-year follow-up. However, the 6-minute walk distance and RVSP continued to improve beyond six months in patients with preoperative PVR >1,000 dynes.s.cm–5 (Figure 3). The range of improvement in 6-minute walk distance was also greater in patients with PVR >1,000 dynes.s.cm–5 compared to patients with PVR <1,000 dynes.s.cm–5.
Survival outcomes
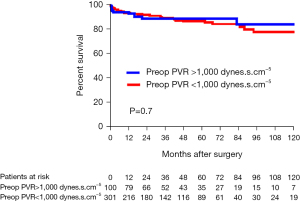
The overall follow-up was similar between patients with PVR greater and lower than 1,000 dynes.s.cm–5 with a mean follow-up of 48±40 and 43±38 months, respectively (P=0.26). Four patients were lost to follow-up within the first year. The overall survival reached 84% at 10 years in patients with PVR >1,000 dynes.s.cm–5 compared to 78% in patients with PVR <1,000 dynes.s.cm–5 (Figure 4). A greater proportion of patients were initiated on targeted PH medical therapy after PEA in patients with preoperative PVR >1,000 dynes.cm–5, reaching 31% on average, compared to 15% in patients with PVR <1,000 dynes.s.cm–5 (P=0.003). In the long-term, the proportion of patients dying from RHF was similar between patients with PVR greater and lower than 1,000 dynes.s.cm–5 (8% vs. 7%, respectively; P=0.8).
Discussion
In this single center analysis, we compared the patients’ characteristics, perioperative outcome and long-term results in patients with preoperative PVR greater and lower than 1,000 dynes.s.cm–5 referred to our institution from PH centers across Canada. We observed that patients with preoperative PVR greater than 1,000 dynes.s.cm–5 were more severely ill with 93% in NYHA functional class III and IV, 29% on home oxygen and 26% requiring hospital admission for RHF. A larger proportion of these patients were also started on targeted PH medical therapy preoperatively.
The postoperative management of patients with severe CTEPH is more complicated due to the risk of pulmonary edema, pneumonia and hemodynamic instability, leading to an operative mortality greater than 10% in most surgical case series (5-8). However, with increasing experience in the postoperative management of patients with severe CTEPH, the operative risks can decrease and the hospital mortality can drop below 5%, even in patients with preoperative PVR >1,000 dynes.s.cm–5 (5).
In our experience, the most challenging patients to manage have been those presenting with a combination of PVR greater than 1,000 dynes.s.cm–5, segmental disease and advanced RHF (7). These patients often have difficulty weaning off CPB and present a particularly high risk of pulmonary edema and hemodynamic instability postoperatively (9). These difficulties are reminiscent of those seen in patients undergoing bilateral lung transplantation for end-stage pulmonary arterial hypertension, where the risk of pulmonary edema is particularly high and the operative mortality is generally greater than 10% (13).
The importance of atrophic remodeling of the left ventricle (LV) with impaired lusitropy and diastolic dysfunction has been emphasized as one of the main risk factors contributing to the development of pulmonary edema after bilateral lung transplantation (13,14). This problem is predominant in patients with advanced RHF due to long-standing precapillary PH and can be overcome by slowly weaning VA ECMO over several days after surgery (15-19). This strategy dramatically reduced the postoperative risks after bilateral lung transplantation for pulmonary arterial hypertension by allowing the LV to progressively adapt to the lower PVR and increased cardiac output (16,17). We used a similar strategy in patients with severe CTEPH who have difficulty separating from CPB after PEA due to advanced RHF, by using central VA ECMO support for a few days after PEA (10). This approach led to a major reduction in the hospital mortality from 10.3% to 1.6% in patients with preoperative PVR >1,000 dynes.s.cm–5.
VA ECMO is associated with significant risks and this option should therefore be used selectively in patients with difficulty weaning from CPB due to either residual PH with hemodynamic instability, or with persistent severe hypoxemia despite inhaled nitric oxide and optimization of mechanical ventilation (10). We observed that the PVR does continue to improve for the first few days after PEA, possibly due to resolution of pulmonary edema, reverse remodeling of the pre- and post-capillary vasculopathy as well as improvement in the left and right ventricular function (9,19,20). Therefore, bridging patients with severe CTEPH and advanced RHF with partial support on VA ECMO for two to three days after PEA in our experience did provide important benefit by both allowing the right and the left ventricles to recover from prolonged CPB and gradual reduction in PVR (10).
This study demonstrates that in the long-term, the results of PEA are comparable in patients with PVR greater and lower than 1,000 dynes.s.cm–5. The ten-year survival is about 80% in both cohorts, including the operative mortality, demonstrating the major benefit of PEA even in the sickest patients, as long as they can overcome the perioperative complications. Patients with preoperative PVR >1,000 dynes.s.cm–5 do, however, more frequently require targeted PH medical therapy after PEA and it is therefore important to monitor these patients in the long-term after surgery. The implementation of BPA in recent years could also provide an opportunity to optimize the benefit of PEA in these patients with more advanced disease.
In conclusion, patients with severe CTEPH are an important group of patients to consider for PEA. These patients often have the most to gain from surgery. The functional improvement is excellent even though up to a third of these patients may require additional targeted PH medical therapy after PEA. The ten-year survival exceeds 80%, which is remarkable considering the poor outcome that these patients would face without PEA.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Perrot M, Gopalan D, Jenkins D, et al. Evaluation and management of patients with chronic thromboembolic pulmonary hypertension - consensus statement from the ISHLT. J Heart Lung Transplant 2021;40:1301-26. [Crossref] [PubMed]
- D'Armini AM, Morsolini M, Mattiucci G, et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2014;148:1005-11; 1012.e1-2; discussion 1011-2.
- Cannon JE, Su L, Kiely DG, et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016;133:1761-71. [Crossref] [PubMed]
- Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141:702-10. [Crossref] [PubMed]
- Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012;94:97-103; discussion 103. [Crossref] [PubMed]
- Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2004;23:637-48. [Crossref] [PubMed]
- de Perrot M, Thenganatt J, McRae K, et al. Pulmonary endarterectomy in severe chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2015;34:369-75. [Crossref] [PubMed]
- Thistlethwaite PA, Kemp A, Du L, et al. Outcomes of pulmonary endarterectomy for treatment of extreme thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2006;131:307-13. [Crossref] [PubMed]
- Condliffe R, Kiely DG, Gibbs JS, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J 2009;33:332-8. [Crossref] [PubMed]
- Abdelnour-Berchtold E, Donahoe L, McRae K, et al. Central venoarterial extracorporeal membrane oxygenation as a bridge to recovery after pulmonary endarterectomy in patients with decompensated right heart failure. J Heart Lung Transplant 2022; in press. [Crossref]
- Tromeur C, Jaïs X, Mercier O, et al. Factors predicting outcome after pulmonary endarterectomy. PLoS One 2018;13:e0198198. [Crossref] [PubMed]
- Thistlethwaite PA, Mo M, Madani MM, et al. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2002;124:1203-11. [Crossref] [PubMed]
- Hoeper MM, Benza RL, Corris P, et al. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J 2019;53:1801906. [Crossref] [PubMed]
- de Perrot M, McRae K. Left ventricular lusitropy and primary graft dysfunction in lung transplantation. J Heart Lung Transplant 2019;38:719-20. [Crossref] [PubMed]
- Hardziyenka M, Campian ME, Reesink HJ, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass: evidence for atrophic remodeling. J Am Coll Cardiol 2011;57:921-8. [Crossref] [PubMed]
- Tudorache I, Sommer W, Kühn C, et al. Lung transplantation for severe pulmonary hypertension--awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015;99:451-8. [Crossref] [PubMed]
- Hoetzenecker K, Schwarz S, Muckenhuber M, et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg 2018;155:2193-2206.e3. [Crossref] [PubMed]
- Levvey BJ, Whitford HM, Williams TJ, et al. Donation After Circulatory Determination of Death Lung Transplantation for Pulmonary Arterial Hypertension: Passing the Toughest Test. Am J Transplant 2015;15:3208-14. [Crossref] [PubMed]
- Gopalan D, Nordgren-Rogberg A, Le EPV, et al. Abnormal Pulmonary Venous Filling: An Adjunct Feature in the Computed Tomography Pulmonary Angiogram Assessment of Chronic Thromboembolic Pulmonary Hypertension. J Am Heart Assoc 2020;9:e018075. [Crossref] [PubMed]
- Dorfmüller P, Günther S, Ghigna MR, et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J 2014;44:1275-88. [Crossref] [PubMed]






