Effects of concomitant coronary artery bypass grafting on early and late mortality in the treatment of post-infarction mechanical complications: a systematic review and meta-analysis
Introduction
Mechanical complications following acute myocardial infarction (AMI) represent rare, but life-threatening events, typically occurring after ST-segment elevation myocardial infarction (STEMI) (1,2). Over the last decades, the wide diffusion of thrombolysis and percutaneous coronary intervention (PCI) for the early treatment of AMI has significantly led to improved survival for coronary artery disease (CAD) and decreased the incidence of such complications (3-5). Nevertheless, they still bear a high in-hospital mortality, even when prompt surgery can be offered (6). Among post-AMI mechanical complications, we recognize ventricular septal rupture (VSR), left-ventricular free-wall rupture (LVFWR) and papillary muscle rupture (PMR) causing acute mitral regurgitation. Being them all early complications of AMI, the proper timing for surgery and the appropriateness of treating the underlying cause through coronary artery bypass grafting (CABG) have been debated. For instance, the advantage of revascularizing necrotic myocardium during high-risk, emergency procedures, remains controversial. Most importantly, there are still conflicting data on whether concomitant CABG (cCABG) provides early and late survival benefit in these patients (7). Thus, we performed a systematic review and meta-analysis of the available literature in order to evaluate the potential survival benefit of cCABG in patients treated surgically for post-AMI mechanical complications.
Methods
Definitions
‘Infarct exclusion’ was defined as the VSR repair technique described by David et al. or any further modification (8). ‘Other techniques’ were defined as any other technique to repair VSR different from the David’s one, including infarct excision (9).
‘Sutureless technique’ was defined as LVFWR repair using collagen sponge, or pericardial patch fixed on the epicardium with glues. ‘Sutured technique’ was defined as LVFWR repair applying sutures to close the myocardial tear or to secure a patch on the epicardium (10).
Early mortality was defined as any death occurred during hospitalization, or within 30 days from surgery. For conflicting data between in-hospital and 30-day mortality, the latter was considered for the survival analysis. Late mortality was defined as any cause-related death after hospital discharge or beyond 30 days from surgery. Reintervention was defined as the need for a further procedure due to failure of repair or rupture recurrence; reoperations for other causes (e.g., bleeding) were not considered.
Literature search strategy
This systematic review and meta-analysis were performed in accordance to the Preferred Recording Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11). A search of the literature on PubMed, EMBASE and the Cochrane Central Register of Controlled Trials was conducted by three independent researchers, to identify eligible studies published between January 2000 and December 2020, using Medical Subject Headings (MeSH) and free-text terms. The keywords were (‘ventricular free-wall rupture’ OR ‘papillary muscle rupture’ OR ‘acute mitral regurgitation’ OR ‘cardiac rupture’ OR ‘ventricular septal rupture’ OR ‘ventricular septal defect’) AND ‘myocardial infarction’. Only publications in English were considered. References of original articles were reviewed manually and cross-checked for other relevant reports that escaped the databases searches.
Eligibility criteria and data extraction
Studies reporting post-operative outcomes of patient receiving repair of LVFWR, VSR or PMR that compared the outcomes of cCABG to the patients who did not undergo concomitant revascularization were included. Exclusion criteria were: (I) animal studies; (II) congenital heart surgery-related studies; (III) studies reporting transcatheter/conservative strategies; (IV) studies not discriminating among mechanical complication types; (V) studies in which outcomes for CABG and non-CABG subgroups were not retrievable. Reviews, case reports or case series reporting <10 cases were not considered. Two independent reviewers (D Ronco and M Matteucci) analyzed the results for eligibility, and extracted studies, as well as relevant patients’ characteristics and outcomes, using an appropriate data collection form. Any divergences were resolved by a third reviewer (C Corazzari). For publications analyzing the same population, the most complete study, according to the variables of interest, was selected.
Pre-operative demographics and assessment were recorded, along with the type of rupture for each complication, namely: oozing or blow-out for LVFWR, anterior/apical or posterior for VSR and anterolateral or posteromedial for PMR. Similarly, intra-operative data and surgical techniques were collected. Main post-operative variables included early and long-term mortality, and major complications (e.g., re-rupture requiring reintervention). Data about salvage/emergent/urgent surgery were not collected because of the high variability in definitions among studies.
Quality assessment and endpoint selection
Two independent reviewers (D Ronco and M Matteucci) assessed the risk of bias at individual study level using the ROBINS-I tool (Risk Of Bias In Not-randomized Studies of Interventions) (12). Any divergences were resolved by a third reviewer (R Lorusso).
The primary endpoint of this meta-analysis was early mortality in the CABG and non-CABG groups. The secondary endpoint was late mortality from any cause. Whenever possible, a 5-year follow-up was considered for each enclosed report, otherwise, the longest available follow-up was selected (at least 1 year).
Statistical analysis
When not available from full-text or supplements, late mortality data were extracted from Kaplan-Meier curves using a dedicated software (Plot Digitizer 2.6.8 for Windows). Review Manager 5.3 software, by the Cochrane Collaboration, was used for statistical computations. Calculation of an overall proportion from studies reporting a single proportion was performed using a meta-analytic approach by means of metaprop function of meta package in R. A logit-transformation was performed as suggested by Warton & Hui; to calculate confidence intervals (CIs) for individual study results, Clopper-Pearson approach was used; inverse variance method was used for data pooling. Subgroup analysis was performed using random effect. DerSimonian-Laird estimator was used to estimate the between-study variance. Total proportion with 95% CI was reported. Heterogeneity was reported as I2. Random-effect model was used to assess difference between early and late death rate among the three different complications. For CABG and non-CABG groups, pooled odds ratios (OR) were reported with 95% CI and a two-tailed P<0.05 was considered statistically significant. Results showing low (I2<50%) to moderate (I2 50–75%) heterogeneity were analyzed by the fixed-effect model, while those with high heterogeneity (I2>75%) were analyzed by the random-effect model. Sensitivity analysis was carried out by successfully excluding low-quality studies to assess the outcome stability. Potential publication bias was evaluated by constructing a funnel plot, with asymmetry suggesting possible publication bias.
Results
The PRISMA flow diagram is presented in Figure 1. After removal of reports not pertinent to the design of the current meta-analysis, 36 studies remained, including 4,321 patients, with a mean age of 69.0±4.0 years and a slight male predominance (58.5%). According to the complication type, patients with VSR accounted for 57.3% of cases, followed by subjects with PMR (37.0%) and LVFWR (5.7%). Pre-operative coronarography was performed in almost all patients (92.2%), most frequently showing single-vessel CAD (54.3%, 1,868/3,440). Pre-operative data are listed in Table 1. Most patients presented with cardiogenic shock before surgery (58.2%). Almost two-thirds of subjects had intra-aortic balloon pump (IABP) placed pre-operatively, and 8.6% required extracorporeal membrane oxygenation (ECMO). VSR was more frequent in anterior/apical portion (60.1%, 627/1,043) and infarct exclusion technique was adopted in 40.5% (246/607) of cases. Of the patients with LVFWR, oozing-type was found in most (58.1%, 115/198) and sutured repair was more frequently performed (55.1%). In PMR group, posteromedial muscle rupture was the commonest (83.8%, 109/130); 79.5% of patients underwent mitral valve replacement (Tables S1-S3).
Table 1
| First author (Ref.) | Year | Type of complication | Number of patients | Mean age (years) | Male (n) | Preoperative angiography (n) | Multiple CAD* (n) | Single CAD (n) | Previous PCI (n) | Previous thrombolysis (n) | Pre-op IABP (n) | Pre-op ECMO (n) | Pre-op shock (n) | Mean pre-op LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abu-Omar (13) | 2012 | VSR | 59 | N/A | 41 | N/A | N/A | N/A | 3 | 30 | 47 | N/A | 33 | N/A |
| Barker (14) | 2003 | VSR | 65 | N/A | 40 | 65 | 46 | 19 | N/A | 31 | 42 | N/A | 35 | N/A |
| Bisoyi (15) | 2020 | VSR | 21 | N/A | 17 | 21 | 7 | 14 | 0 | 0 | 20 | 0 | 6 | N/A |
| Bouma (16) | 2014 | PMR | 48 | 64.9 | 34 | 48 | 25 | 23 | 12 | N/A | 21 | N/A | 31 | N/A |
| Cinq-Mars (17) | 2016 | VSR | 34 | 69 | 19 | 34 | 18 | 16 | N/A | 11 | 28 | 0 | 24 | 44 |
| Dogra (18) | 2019 | VSR | 35 | 61 | 19 | 35 | N/A | N/A | 8 | 12 | 16 | 1 | 15 | 33 |
| Formica (19) | 2018 | FWR | 35 | 68.3 | 25 | 27 | N/A | N/A | 9 | N/A | 14 | 12 | 12 | 45.7 |
| Fukushima (20) | 2010 | VSR | 68 | 66.4 | 49 | 65 | 45 | 20 | 3 | 0 | 28 | 1 | 8 | N/A |
| Furukawa (21) | 2000 | VSR | 12 | 71.3 | 6 | 12 | 7 | 5 | 6 | 0 | 9 | N/A | 9 | N/A |
| Huang (22) | 2015 | VSR | 47 | 68.9 | 28 | 47 | 31 | 16 | 17 | 12 | 34 | 6 | 19 | 45.8 |
| Iemura (23) | 2001 | FWR | 17 | 65.4 | 14 | 15 | N/A | N/A | 7 | 12 | N/A | N/A | 11 | N/A |
| Jeppsson (24) | 2005 | VSR | 189 | 69 | 119 | 148 | N/A | N/A | N/A | 64 | 91 | 0 | N/A | N/A |
| Kacer (25) | 2020 | FWR | 19 | N/A | 12 | N/A | N/A | N/A | N/A | N/A | 1 | 0 | 3 | N/A |
| Khan (26) | 2018 | VSR | 31 | 57.1 | 21 | 31 | 6 | 25 | N/A | N/A | 13 | 0 | 7 | 38.4 |
| Kilic (27) | 2020 | PMR | 1,342 | 65.6 | 911 | 1,171 | 792 | 379 | N/A | N/A | 764 | 41 | 759 | 53.1 |
| Kim (28) | 2015 | VSR | 23 | 68 | 11 | 23 | 7 | 14 | 4 | N/A | 19 | 1 | N/A | 42.5 |
| Labrousse (29) | 2002 | VSR | 85 | 69 | 51 | 72 | N/A | N/A | N/A | N/A | 81 | N/A | 16 | N/A |
| Lorusso (30) | 2008 | PMR | 126 | 66.5 | 40 | 83 | N/A | N/A | N/A | N/A | 50 | N/A | 94 | N/A |
| Malhotra (31) | 2017 | VSR | 40 | 61.6 | 26 | 40 | 22 | 18 | N/A | N/A | 40 | N/A | N/A | 37 |
| Mantovani (32) | 2002 | FWR | 17 | 68 | 11 | 16 | 11 | 5 | 3 | 3 | 7 | 0 | N/A | N/A |
| Mantovani (33) | 2006 | VSR | 50 | 66 | 26 | 49 | 24 | 25 | N/A | 15 | 28 | 0 | N/A | N/A |
| Martinelli (34) | 2003 | VSR | 12 | 64.4 | 10 | 12 | 7 | 5 | N/A | N/A | 6 | N/A | N/A | 34.6 |
| Matteucci (35) | 2020 | FWR | 140 | 69.4 | 91 | 104 | 64 | 40 | 48 | 10 | 51 | 16 | 100 | 41.7 |
| Okada (36) | 2005 | VSR | 10 | 72 | 3 | 10 | 2 | 8 | 4 | N/A | 10 | 1 | 1 | 43.2 |
| Okamura (37) | 2019 | FWR | 35 | 71.5 | 21 | 32 | 6 | 29 | 19 | N/A | 13 | 4 | 25 | N/A |
| Ozkara (38) | 2005 | VSR | 20 | 62.1 | 15 | 17 | 7 | 10 | N/A | N/A | 19 | N/A | 4 | N/A |
| Pang (39) | 2013 | VSR | 38 | 65.7 | 20 | 36 | 22 | 14 | N/A | 11 | 37 | 0 | 26 | 39.7 |
| Pojar (40) | 2018 | VSR | 39 | 68.4 | 19 | 39 | 18 | 21 | 8 | 4 | 17 | 1 | N/A | 47.2 |
| Russo (6) | 2008 | PMR | 54 | 70 | 40 | 53 | 36 | 17 | N/A | N/A | 40 | N/A | 49 | 56 |
| Sakaguchi (41) | 2019 | VSR | 1,397 | 74.1 | 671 | 1,397 | 303 | 1,094 | 508 | N/A | 1,075 | 224† | 859 | N/A |
| Schroeter (42) | 2013 | PMR | 28 | 63.4 | 22 | 28 | N/A | N/A | 9 | N/A | 12 | N/A | 15 | 50.2 |
| Takahashi (43) | 2015 | VSR | 52 | 67 | 26 | 52 | 33 | 17 | 7 | 0 | 20 | N/A | 30 | N/A |
| Thiele (44) | 2003 | VSR | 20 | 68.5 | 12 | 20 | 11 | 9 | 15 | N/A | 20 | 3 | 9 | 42 |
| Wiemers (45) | 2012 | VSR | 10 | 65.3 | 5 | 8 | 7 | 1 | 3 | 6 | 6 | N/A | 1 | N/A |
| Yalçinkaya (46) | 2016 | VSR | 63 | 67.2 | 35 | 63 | N/A | N/A | 19 | 23 | 57 | N/A | N/A | 45.2 |
| Yam (47) | 2013 | VSR | 40 | N/A | 16 | 38 | 14 | 24 | N/A | N/A | 32 | 0 | 11 | 56 |
| Total | 4,321 | 69.0±4.0 | 2,526 | 3,911 | 1,571 | 1,868 | 712 | 244 | 2,768 | 311 | 2,212 | 44.2±6.6 |
*, including patients with left main CAD only; †, including preoperative and intraoperative ECMO. CAD, coronary artery disease; ECMO, extracorporeal membrane oxygenation; FWR, free-wall rupture; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; N/A, not-available; PCI, percutaneous coronary intervention; PMR, papillary muscle rupture; VSR, ventricular septal rupture.
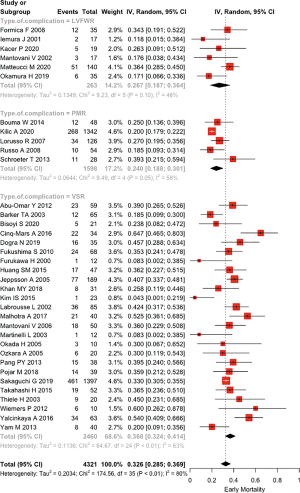
Operative and post-operative data are shown in Table 2. Concomitant CABG was performed in 49.0% of patients (43.8% in VSR, 31.7% in LVFWR and 59.7% in PMR). The pooled early mortality was 32.6% (28.5–36.9%) with I2=79.9% (72.8–85.2%); in VSR patients it was 36.8% (32.4–41.6%), while in subjects with LVFWR it was 26.6% (18.7–36.4%) and in patients with PMR it was 24.0% (18.9–30.1%), P=0.0023 (Figure 2). Among individuals who survived the surgery, the commonest cause of in-hospital death was low-cardiac-output syndrome (34.7%, 151/435). Reintervention for residual or recurrent rupture was required in 8.4% of cases.
Table 2
| First author (Ref.) | Mean CPB time (min) | Mean aortic cross-clamp time (min) | Concomitant CABG (n) | Post-op IABP (n) | Post-op ECMO (n) | Reintervention (n) | Early mortality (n) | Causes of death (n)* | Late mortality (n) | Mean follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abu-Omar (13) | 110 | 58 | 44 | N/A | N/A | N/A | 23 | N/A | 12 | 5 |
| Barker (14) | N/A | N/A | 42 | 42 | 0 | N/A | 12 | N/A | 16 | 2.4 [4] |
| Bisoyi (15) | N/A | N/A | 17 | 1 | 0 | 0 | 5 | LCOS (n=4) | 0 | 5 |
| Bouma (16) | 178 | 98 | 24 | 24 | 0 | 0 | 12 | LCOS (n=6), rupture (n=2), bleeding (n=2) | N/A | N/A |
| Cinq-Mars (17) | 141 | 94 | 15 | 2 | 1 | N/A | 22 | LCOS (n=18), MOF (n=2), sepsis (n=1), respiratory (n=1) | 6 | [12] |
| Dogra (18) | 172 | 116 | 22 | 0 | 0 | N/A | 16 | LCOS (n=15), unknown (n=1) | 1 | [5] |
| Formica (19) | 121.4 | 52.3 | 15 | 10 | 11 | 0 | 12 | MOF (n=2), sepsis (n=1), CVA (n=5), arrhythmia (n=1), bowel ischemia (n=2) | 6 | 12.6 |
| Fukushima (20) | N/A | N/A | 48 | N/A | 1 | 9‡ | 24 | LCOS (n=13), sepsis (n=2), arrhythmia (n=1), bleeding (n=1), anemia (n=2) | 16 | 9.2 |
| Furukawa (21) | 127.5 | 60.1 | 5 | 9 | N/A | 0 | 0 | 0 | 2 | 5 |
| Huang (22) | 193.9 | 113 | 27 | N/A | N/A | N/A | 17 | N/A | 11 | 8.3 [6] |
| Iemura (23) | N/A | 60.1 | 8 | 15 | N/A | 0 | 2 | LCOS (n=2) | 1 | [1.6] |
| Jeppsson (24) | N/A | N/A | 119 | 55 | 0 | 21 | 77 | N/A | 38 | 2.4 [8] |
| Kacer (25) | N/A | N/A | 7 | 4 | 1 | 2 | 5 | Rupture (n=1) | 0 | [3.8] |
| Khan (26) | 120 | 61.7 | 18 | N/A | 0 | 0 | 8 | N/A | 10 | [6] |
| Kilic (27) | 162.4 | 111.4 | 796 | N/A | N/A | 137 | 268 | N/A | N/A | N/A |
| Kim (28) | 194.4 | 150.1 | 17 | N/A | N/A | 3 | 1 | Right ventricular failure (n=1) | 5 | 2.2 |
| Labrousse (29) | N/A | N/A | 40 | N/A | 0 | 3 | 36 | LCOS (n=22), rupture (n=4), CVA (n=3), arrhythmia (n=2), AKI (n=2), IABP complication (n=2), other (n=1) | 31 | 7.2 |
| Lorusso (30) | N/A | N/A | 73 | N/A | N/A | N/A | 34 | N/A | 29 | [5] |
| Malhotra (31) | 159 | 105.4 | 28 | N/A | N/A | 1 | 21 | LCOS (n=15), sepsis (n=4) | 2 | 1.7 |
| Mantovani (32) | 108 | 61 | 11 | N/A | 0 | 1 | 3 | LCOS (n=1), MOF (n=1), AKI (n=1) | 3 | 3.8 |
| Mantovani (33) | N/A | 101 | 25 | N/A | 0 | 4 | 18 | N/A | 20 | [15] |
| Martinelli (34) | N/A | N/A | 7 | 6 | N/A | 3 | 0 | 0 | 1 | 2.5 |
| Matteucci (35) | 104.4 | 67.1 | 34 | 67 | 11 | 7 | 51 | LCOS (n=22), rupture (n=9), CVA (n=8), AKI (n=2), Sepsis (n=1), bowel ischemia (n=1) | N/A | N/A |
| Okada (36) | 152.5 | 88.5 | 1 | N/A | N/A | 2 | 3 | LCOS (n=2), MOF (n=1) | N/A | N/A |
| Okamura (37) | 0† | 0† | 3 | N/A | N/A | 5 | 6 | LCOS (n=1), rupture (n=1), arrhythmia (n=1), pneumonia (n=3) | 11 | [10] |
| Ozkara (38) | 98.7 | 62 | 14 | N/A | N/A | 3 | 6 | MOF (n=3), CVA (n=1), arrhythmia (n=2) | 1 | 5.8 |
| Pang (39) | 152 | 82 | 19 | N/A | 1 | 1 | 15 | MOF (n=1), unknown (n=13) | 8 | [10] |
| Pojar (40) | 146.3 | 91.8 | 12 | N/A | N/A | 0 | 14 | N/A | 10 | [5] |
| Russo (6) | 89 | N/A | 42 | 39 | N/A | 0 | 10 | LCOS (n=1), rupture (n=2), MI (n=3), other (n=2) | 28 | 6.4 |
| Sakaguchi (41) | 198 | 124 | 475 | 125 | N/A | N/A | 461 | N/A | N/A | N/A |
| Schroeter (42) | 151 | 66 | 19 | 20 | 9 | N/A | 11 | N/A | N/A | N/A |
| Takahashi (43) | 161.5 | 83.1 | 33 | N/A | N/A | 4 | 19 | LCOS (n=19) | 8 | 7.8 [5] |
| Thiele (44) | N/A | N/A | 6 | N/A | 6 | N/A | 9 | MOF (n=9) | 1 | 3.6 |
| Wiemers (45) | 157 | 115 | 5 | N/A | N/A | 1 | 6 | LCOS (n=4) | 0 | 3.4 |
| Yalçinkaya (46) | 102.7 | 65.1 | 38 | 48 | N/A | 2 | 34 | LCOS (n=4), rupture (n=2), unknown (n=28) | 10 | [5] |
| Yam (47) | 117 | 87 | 8 | N/A | 0 | 2 | 8 | LCOS (n=2), sepsis (n=4), CVA (n=1), arrhythmia (n=1) | 15 | 5.2 [10] |
| Total | 170.3±32.3 | 109.3±25.6 | 2,117 | 467 | 41 | 211 | 1,269 | – | 302 | 5.2±2.8 |
*, excluding intraoperative deaths; †, all surgeries performed off-pump; ‡, one reintervention performed percutaneously. In parentheses: data extracted from Kaplan-Meier curves. In square brackets: years of follow-up considered for data extraction. AKI, acute kidney injury; CABG, coronary artery bypass grafting; CPB, cardio-pulmonary bypass; CVA, cerebrovascular accident; LCOS, low-cardiac-output syndrome; MI, myocardial infarction; MOF, multi-organ failure.
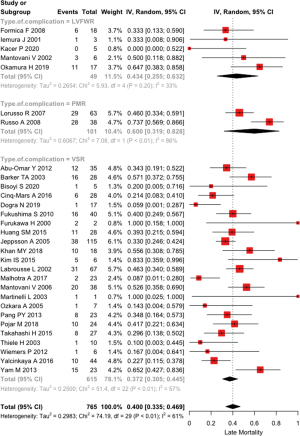
The pooled late mortality was 40.0% (33.5–46.9%) with I2=60.9% (41.8–73.7%); in VSR patients it was 37.2% (30.5–44.9%), while in subjects with LVFWR it was 43.4% (25.5–63.3%) and in patients with PMR it was 59.9% (31.9–82.7%), P=0.2870 (Figure 3). Mean follow up was 5.2±2.8 years.
Primary endpoint
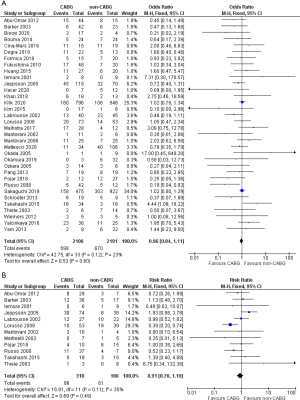
In the early mortality analysis, there was no difference between CABG and non-CABG groups (OR 0.96; 95% CI:0.84–1.11; P=0.60, I2=23%; Figure 4A). Single complication subgroup analysis accordingly showed that cCABG didn’t affect early mortality in any of the three types of mechanical complication: VSR (OR 1.00; 95% CI: 0.84–1.19; P=0.98, I2=26%; Figure S1A); PMR (OR 0.94; 95% CI: 0.74–1.20; P=0.62, I2=39%; Figure S1B); and LVFWR (OR 0.73; 95% CI: 0.40–1.33; P=0.30, I2=0%; Figure S1C).
Secondary endpoint
Long-term survival data of 12 studies could be analyzed, including 496 patients, with a follow-up ranging from 1.5 to 7.2 years. Late mortality was 31.0% in the CABG versus 32.8% in the non-CABG group, hence showing no significant difference (RR 0.91, 95% CI: 0.70–1.19, P=0.49, I2=35%; Figure 4B). However, the single complication analysis showed, for VSR patients, a lower late mortality in the non-CABG group, although not statistically significant (RR 1.24; 95% CI: 0.89–1.73; P=0.20, I2=0%). Conversely, LVFWR patients showed a slight trend toward a better, but not significant, late survival with cCABG (RR 0.65; 95% CI: 0.11–3.71; P=0.63; I2=0%). Finally, late mortality appeared significantly lower with cCABG in PMR (RR 0.42; 95% CI: 0.25–0.70; P=0.001; I2=0%) (Figure S2).
Discussion
The current meta-analysis showed that cCABG in the setting of surgery for post-infarction mechanical complications is not associated with a lower early or late mortality.
The value of cCABG during surgery for post-infarction mechanical complications remains a matter of debate. Despite representing a treatment for the underlying cause of cardiac rupture, the effects of surgical revascularization on early and late mortality have not been clarified yet (7,35).
VSR, LVFWR and PMR are well-known AMI complications, usually occurring within one week of the ischemic episode (1,4). Elbadawi et al. recently analyzed the U.S. National Inpatient Sample database, showing that, from 2003 to 2015, among almost 9 million AMI patients, the incidence of VSR, LVFWR and PMR was 0.25%, 0.02% and 0.06%, respectively (1). Over the last decades, the incidence of cardiac rupture significantly decreased, mainly due to the introduction of early percutaneous revascularization, proving its beneficial effect especially in STEMI patients, who are more at risk for such ominous complications (2-5,18). This important aspect also emerged in the ongoing COVID-19 pandemics, when delayed and difficult access to appropriate treatment has led to a remarkably increased mortality for AMI, paralleled by an increased occurrence of its complications (48).
Thirty-day mortality for these patients can be as high as 90% if left untreated, and urgent surgery with prompt hemodynamic stabilization is often demanded (16,49,50). Nevertheless, surgical in-hospital mortality still ranges from 12% to 60%, depending on reports and type of complication (6,8,17,19). Considering such a high mortality, some authors argued that increasing operative risk and cardio-pulmonary bypass time to restore blood supply to infarcted myocardium wouldn’t be justified by a questionable survival advantage (50,51). Our interest was to evaluate if cCABG in these patients is favored for early mortality and if the myocardial protection it provides is maintained beyond the early peri-operative phase.
This meta-analysis shows that cCABG in surgery for post-AMI mechanical complications can be performed safely without significantly increasing early and late mortality, similarly to what Horan et al. recently reported for VSR patients only (7). However, it should be noted that our analysis didn’t consider percutaneous revascularization, that was carried out in 956 patients and is usually reported to improve survival (4,5). Indeed, Dogra et al. identified early thrombolysis as the most important prognostic factor in VSR, decreasing the risk of cardiac rupture (18). Differently, Bouma et al. reported that PCI had no effect on long-term survival in PMR patients (52). While considering the favorable contribution of early percutaneous revascularization to risk reduction of cardiac rupture, some authors described that this complication might be accelerated with thrombolysis, causing myocardial hemorrhage during the ‘lytic state’ of AMI (53). A reduced AMI-to-VSR time-frame in subjects who underwent thrombolysis was also observed in the GUSTO-I trial (4). In our population the mean time from AMI to rupture was 3.5±1.2 days.
When cardiac rupture occurs, it’s not always possible to perform PCI nor just coronarography either, thereby precluding any possibility of surgical revascularization (20,24,54). Indeed, most patients with post-AMI mechanical complications are admitted to hospital in poor hemodynamic conditions or even in cardiac arrest, as demonstrated by the 58.2% of subjects developing cardiogenic shock pre-operatively and by the 64.3% and 8.6% requiring IABP and ECMO support, respectively (6,18,26,30,41,44). Therefore, emergent surgery might be necessary, thus making any pre-operative assessment unfeasible and time-consuming (55). Moreover, Skillington et al. pointed out the potentially harmful effect of coronarography on such unstable patients (33,39,47,55). However, many others supported the routine execution of pre-operative coronarography in all patients who don’t need a salvage procedure and can be effectively stabilized (20,24,50,51,56). Furthermore, a discussion on the possible advantages of an expanded use of mechanical circulatory supports to achieve patients stabilization has emerged in the last years, in order to complete diagnostic workup and bring the patients to an elective procedure rather than an emergent surgery (57).
Cardiac rupture usually occurs in single-vessel disease (more frequently LAD) and during the first ischemic episode (24,31,39,47). In the current study, 54.3% of patients had single-vessel CAD (4,7,31). The role of lacking collateral circulation in its pathogenesis may also explain why 83.8% of PMR patients had posteromedial muscle rupture, being it more sensitive to ischemia, because it’s usually supplied by terminal branches (58).
In case of single-vessel CAD, PCI of the infarct-related artery (IRA) or thrombolysis is usually performed (22). However, in reports where multivessel CAD was more frequent, CABG was performed more often, showing most of its possible advantage (14). This may explain the survival difference in Barker’s and Jeppsson’s reports, the latter observing that the extent of CAD predicts late mortality, differently from Yalçınkaya et al. (14,24,46).
Concomitant CABG plays a different role in the IRA compared to other stenotic vessels (7). Indeed, some authors argued that revascularization of a coronary supplying infarcted myocardium is of little use, with the disadvantages outweighing the advantages (46,47,50,51). Differently, other reports showed that IRA revascularization may improve early and long-term survival by providing ischemic border perfusion and better control of possible arrhythmias (51). Prêtre et al. considered the IRA revascularization only in presence of a large septal or a significant collateral branch reaching viable myocardium (50).
CABG role in patients without viable myocardium has been extensively studied. The STICHES trial showed no significant difference on long-term survival in patients undergone CABG with pre-operative viable versus non-viable myocardium (59).
In multivessel CAD, non-IRA revascularization is considered logical by most authors (14,35,39,60,61). Although Yam et al. observed no survival benefit of cCABG in multivessel disease (47), Lundblad et al. highlighted that revascularization impacts more significantly on extensive CAD, pointing out the importance of complete revascularization (51). Similarly, Takahashi et al. identified incomplete revascularization as an independent risk for early mortality, and Mantovani et al. reported a higher mortality for patients left with myocardium at risk of ischemia (33,43). Other authors reported a long-term survival benefit for patients receiving total revascularization, probably for a better myocardial recovery provided by collateral bloodflow (7,22).
Concomitant CABG increases the surgical risk, reflected in EuroSCORE II as an independent predictor of mortality (22,33). Nevertheless, our meta-analysis showed no difference in early mortality between CABG and non-CABG, suggesting that concomitant revascularization doesn’t negatively affect patient survival, probably by controlling the added risk of CAD (14,29).
Most studies included in our meta-analysis showed no difference in early mortality between CABG and non-CABG groups, while some authors reported a better survival for patients undergoing CABG (6,14,40,54,62). Takahashi et al. observed a higher early mortality for patients undergoing cCABG, explaining such results with a more severe CAD and ventricular dysfunction in that group of patients (43).
Analyzing the three types of complication, in VSR and PMR no difference was found between treatment groups, while LVFWR patients showed a trend slightly favoring cCABG, although not statistically significant. It should be noted, however, that each type of complication has different surgical implications. Indeed, surgery for PMR often requires standard mitral valve repair/replacement and as a result, cCABG is less technically demanding, probably contributing to a better outcome compared to the other conditions, although controversial results have been reported (6,27,63). In VSR, complete revascularization is not always possible, because most techniques require ventricular opening on the infarct area and the closing suture often entraps the culprit vessel that can therefore seldom be grafted (8,51). Similarly, in LVFWR both sutureless and sutured techniques make part of the ventricular wall inaccessible (10). As a matter of fact, Matteucci et al. suggested that the real effect of cCABG in LVFWR may be underestimated by the relatively low number of patients undergoing surgical revascularization, thereby possibly justifying the lack of cCABG impact on early mortality (35).
Despite few studies reporting a survival benefit for cCABG, all the authors recommend cCABG whenever possible (14,29,35,43,50,63). Randomized trials would be required to draw better conclusions, but it would be unethical to prevent patients needing CABG from receiving the appropriate treatment (33). The current meta-analysis also showed no survival difference on late mortality between treatment groups. However, in VSR patients there was a trend favoring non-CABG although not statistically significant, differently from the recent observations of Horan et al. (7). This result is strongly influenced by the population of Jeppsson et al. who reported a higher late mortality in cCABG patients (24). Conversely, Barker et al. reported a better long-term survival for cCABG patients after adjustment, although the crude data we analyzed showed no significant difference (14). Other reports showed no difference between treatment groups (29,40,60).
For LVFWR and PMR patients, late mortality data were available in four studies only. In LVFWR, a non-significant trend favoring cCABG was found, with Mantovani et al. suggesting a CABG contribution to long-term angina-free survival (32). For PMR, cCABG appeared to provide statistically better late survival (30).
Therefore, from the late-mortality results of this meta-analysis it seems reasonable that cCABG could represent a protective factor preventing patients with a more severe condition from further worsening (14,39). Nevertheless, Sulzgruber et al. observed that patients surviving the peri-operative period after cardiac rupture show a long-term mortality comparable to other AMI patients (64). However, the small sample size of most reports and the low incidence of these conditions make it difficult to draw definitive conclusions and advocate larger studies to increase the evidence on this topic.
Limitations
This study contains all the biases inherent to systematic reviews. Particularly, the major limitation is the quality of the included studies (mostly retrospective, with more than half of them analyzing small sample sizes). The included reports may account for a high risk of publication and selection bias. Moreover, some missing data on long-term follow-up were extracted from Kaplan-Meier curves. Data from two national registries were included, but accurate study selection eliminated the potential risk of patient overlapping. We also acknowledge the lack of some useful information for outcome analysis, such as data about emergent/urgent operation and causes of late death. Finally, we couldn’t collect data about the need for further revascularization after hospital discharge.
Conclusions
Post-AMI mechanical complications represent rare, but life-threatening events. Surgical treatment constitutes the standard of care, however in-hospital mortality remains high. Concomitant CABG may represent an effective treatment for the underlying cause of these complications, especially in multivessel CAD, albeit increasing the surgical risk. This meta-analysis showed no significant difference between CABG and non-CABG groups in both early and late mortality, with some distinctions among different mechanical complications. However, we believe that cCABG could provide early and long-term advantage by preventing the added risk of CAD progression and should therefore be performed whenever feasible in these patients. Further and dedicated studies are warranted to evaluate the safety and effectiveness of cCABG in this setting.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: RL is a consultant for Medtronic, Getinge and LivaNova, and Member of the advisory board of Eurosets and Fresenius/Xenios. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv 2019;12:1825-36. [Crossref] [PubMed]
- López-Sendón J, Gurfinkel EP, Lopez de Sa E, et al. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. Eur Heart J 2010;31:1449-56. [Crossref] [PubMed]
- Figueras J, Alcalde O, Barrabés JA, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008;118:2783-9. [Crossref] [PubMed]
- Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000;101:27-32. [Crossref] [PubMed]
- French JK, Hellkamp AS, Armstrong PW, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am J Cardiol 2010;105:59-63. [Crossref] [PubMed]
- Russo A, Suri RM, Grigioni F, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation 2008;118:1528-34. [Crossref] [PubMed]
- Horan DP, O'Malley TJ, Weber MP, et al. Repair of ischemic ventricular septal defect with and without coronary artery bypass grafting. J Card Surg 2020;35:1062-71. [Crossref] [PubMed]
- David TE, Armstrong S. Surgical repair of postinfarction ventricular septal defect by infarct exclusion. Semin Thorac Cardiovasc Surg 1998;10:105-10. [Crossref] [PubMed]
- Daggett WM, Burwell LR, Lawson DW, et al. Resection of acute ventricular aneurysm and ruptured interventricular septum after myocardial infarction. N Engl J Med 1970;283:1507-8. [Crossref] [PubMed]
- Matteucci M, Fina D, Jiritano F, et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care 2019;8:379-87. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Abu-Omar Y, Bhinda P, Choong CK, et al. Survival after surgical repair of ischemic ventricular septal rupture. Asian Cardiovasc Thorac Ann 2012;20:404-8. [Crossref] [PubMed]
- Barker TA, Ramnarine IR, Woo EB, et al. Repair of post-infarct ventricular septal defect with or without coronary artery bypass grafting in the northwest of England: a 5-year multi-institutional experience. Eur J Cardiothorac Surg 2003;24:940-6. [Crossref] [PubMed]
- Bisoyi S, Jagannathan U, Dash AK, et al. Decision making, management, and midterm outcomes of postinfarction ventricular septal rupture: Our experience with 21 patients. Ann Card Anaesth 2020;23:471-6. [Crossref] [PubMed]
- Bouma W, Wijdh-den Hamer IJ, Koene BM, et al. Predictors of in-hospital mortality after mitral valve surgery for post-myocardial infarction papillary muscle rupture. J Cardiothorac Surg 2014;9:171. [Crossref] [PubMed]
- Cinq-Mars A, Voisine P, Dagenais F, et al. Risk factors of mortality after surgical correction of ventricular septal defect following myocardial infarction: Retrospective analysis and review of the literature. Int J Cardiol 2016;206:27-36. [Crossref] [PubMed]
- Dogra N, Puri GD, Thingnam SKS, et al. Early thrombolysis is associated with decreased operative mortality in postinfarction ventricular septal rupture. Indian Heart J 2019;71:224-8. [Crossref] [PubMed]
- Formica F, Mariani S, Singh G, et al. Postinfarction left ventricular free wall rupture: a 17-year single-centre experience. Eur J Cardiothorac Surg 2018;53:150-6. [Crossref] [PubMed]
- Fukushima S, Tesar PJ, Jalali H, et al. Determinants of in-hospital and long-term surgical outcomes after repair of postinfarction ventricular septal rupture. J Thorac Cardiovasc Surg 2010;140:59-65. [Crossref] [PubMed]
- Furukawa H, Tsuchiya K, Ogata K, et al. Surgical repair of postinfarction ventricular septal rupture. Jpn J Thorac Cardiovasc Surg 2000;48:199-204. [Crossref] [PubMed]
- Huang SM, Huang SC, Wang CH, et al. Risk factors and outcome analysis after surgical management of ventricular septal rupture complicating acute myocardial infarction: a retrospective analysis. J Cardiothorac Surg 2015;10:66. [Crossref] [PubMed]
- Iemura J, Oku H, Otaki M, et al. Surgical strategy for left ventricular free wall rupture after acute myocardial infarction. Ann Thorac Surg 2001;71:201-4. [Crossref] [PubMed]
- Jeppsson A, Liden H, Johnsson P, et al. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg 2005;27:216-21. [Crossref] [PubMed]
- Kacer P, Adamkova V, Hubacek JA, et al. Post-infarction left ventricular free wall rupture: 12-years experience from the Cardiac Centre of the Institute of Clinical and Experimental Medicine in Prague, Czech Republic. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2021;165:408-15. [Crossref] [PubMed]
- Khan MY, Waqar T, Qaisrani PG, et al. Surgical Repair of post-infarction ventricular septal rupture: Determinants of operative mortality and survival outcome analysis. Pak J Med Sci 2018;34:20-6. [Crossref] [PubMed]
- Kilic A, Sultan I, Chu D, et al. Mitral Valve Surgery for Papillary Muscle Rupture: Outcomes in 1,342 Patients from the STS Database. Ann Thorac Surg 2020;110:1975-81. [Crossref] [PubMed]
- Kim IS, Lee JH, Lee DS, et al. Surgical Outcomes of a Modified Infarct Exclusion Technique for Post-Infarction Ventricular Septal Defects. Korean J Thorac Cardiovasc Surg 2015;48:381-6. [Crossref] [PubMed]
- Labrousse L, Choukroun E, Chevalier JM, et al. Surgery for post infarction ventricular septal defect (VSD): risk factors for hospital death and long term results. Eur J Cardiothorac Surg 2002;21:725-31; discussion 731-2. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, De Cicco G, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573-82. [Crossref] [PubMed]
- Malhotra A, Patel K, Sharma P, et al. Techniques, Timing & Prognosis of Post Infarct Ventricular Septal Repair: a Re-look at Old Dogmas. Braz J Cardiovasc Surg 2017;32:147-55. [Crossref] [PubMed]
- Mantovani V, Vanoli D, Chelazzi P, et al. Post-infarction cardiac rupture: surgical treatment. Eur J Cardiothorac Surg 2002;22:777-80. [Crossref] [PubMed]
- Mantovani V, Mariscalco G, Leva C, et al. Surgical repair of post-infarction ventricular septal defect: 19 years of experience. Int J Cardiol 2006;108:202-6. [Crossref] [PubMed]
- Martinelli L, Dottori V, Caputo E, et al. Early closure of postinfarction ventricular septal defects. Ital Heart J 2003;4:325-8. [PubMed]
- Matteucci M, Kowalewski M, De Bonis M, et al. Surgical Treatment of Post-Infarction Left Ventricular Free-Wall Rupture: A Multicenter Study. Ann Thorac Surg 2021;112:1186-92. [Crossref] [PubMed]
- Okada H, Nishida M, Murakami M, et al. Surgical treatment for complications of acute myocardial infarction. Jpn J Thorac Cardiovasc Surg 2005;53:74-7. [Crossref] [PubMed]
- Okamura H, Kimura N, Mieno M, et al. Sutureless repair for postinfarction left ventricular free wall rupture. J Thorac Cardiovasc Surg 2019;158:771-7. [Crossref] [PubMed]
- Ozkara A, Cetin G, Mert M, et al. Postinfarction ventricular septal rupture: surgical intervention and risk factors influencing hospital mortality. Acta Cardiol 2005;60:213-7. [Crossref] [PubMed]
- Pang PY, Sin YK, Lim CH, et al. Outcome and survival analysis of surgical repair of post-infarction ventricular septal rupture. J Cardiothorac Surg 2013;8:44. [Crossref] [PubMed]
- Pojar M, Harrer J, Omran N, et al. Surgical treatment of postinfarction ventricular septal defect: risk factors and outcome analysis. Interact Cardiovasc Thorac Surg 2018;26:41-6. [Crossref] [PubMed]
- Sakaguchi G, Miyata H, Motomura N, et al. Surgical Repair of Post-Infarction Ventricular Septal Defect - Findings From a Japanese National Database. Circ J 2019;83:2229-35. [Crossref] [PubMed]
- Schroeter T, Lehmann S, Misfeld M, et al. Clinical outcome after mitral valve surgery due to ischemic papillary muscle rupture. Ann Thorac Surg 2013;95:820-4. [Crossref] [PubMed]
- Takahashi H, Arif R, Almashhoor A, et al. Long-term results after surgical treatment of postinfarction ventricular septal rupture. Eur J Cardiothorac Surg 2015;47:720-4. [Crossref] [PubMed]
- Thiele H, Lauer B, Hambrecht R, et al. Short- and long-term hemodynamic effects of intra-aortic balloon support in ventricular septal defect complicating acute myocardial infarction. Am J Cardiol 2003;92:450-4. [Crossref] [PubMed]
- Wiemers P, Harvey R, Khatun M, et al. Management and midterm outcomes of post-infarction ventricular septal defect. Asian Cardiovasc Thorac Ann 2012;20:663-8. [Crossref] [PubMed]
- Yalçınkaya A, Lafçı G, Diken Aİ, et al. Early Mortality and Long-term Survival after Repair of Post-infarction Ventricular Septal Rupture: An Institutional Report of Experience. Heart Lung Circ 2016;25:384-91. [Crossref] [PubMed]
- Yam N, Au TW, Cheng LC. Post-infarction ventricular septal defect: surgical outcomes in the last decade. Asian Cardiovasc Thorac Ann 2013;21:539-45. [Crossref] [PubMed]
- De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083-8. [Crossref] [PubMed]
- Carnero-Alcázar M, Alswies A, Pérez-Isla L, et al. Short-term and mid-term follow-up of sutureless surgery for postinfarction subacute free wall rupture. Interact Cardiovasc Thorac Surg 2009;8:619-23. [Crossref] [PubMed]
- Prêtre R, Ye Q, Grünenfelder J, et al. Role of myocardial revascularization in postinfarction ventricular septal rupture. Ann Thorac Surg 2000;69:51-5. [Crossref] [PubMed]
- Lundblad R, Abdelnoor M, Geiran OR, et al. Surgical repair of postinfarction ventricular septal rupture: risk factors of early and late death. J Thorac Cardiovasc Surg 2009;137:862-8. [Crossref] [PubMed]
- Bouma W, Wijdh-den Hamer IJ, Koene BM, et al. Long-term survival after mitral valve surgery for post-myocardial infarction papillary muscle rupture. J Cardiothorac Surg 2015;10:11. [Crossref] [PubMed]
- Becker RC, Charlesworth A, Wilcox RG, et al. Cardiac rupture associated with thrombolytic therapy: impact of time to treatment in the Late Assessment of Thrombolytic Efficacy (LATE) study. J Am Coll Cardiol 1995;25:1063-8. [Crossref] [PubMed]
- Ronco D, Matteucci M, Kowalewski M, et al. Surgical Treatment of Postinfarction Ventricular Septal Rupture. JAMA Netw Open 2021;4:e2128309. [Crossref] [PubMed]
- Skillington PD, Davies RH, Luff AJ, et al. Surgical treatment for infarct-related ventricular septal defects. Improved early results combined with analysis of late functional status. J Thorac Cardiovasc Surg 1990;99:798-808. [Crossref] [PubMed]
- Dalrymple-Hay MJ, Monro JL, Livesey SA, et al. Postinfarction ventricular septal rupture: the Wessex experience. Semin Thorac Cardiovasc Surg 1998;10:111-6. [Crossref] [PubMed]
- Ronco D, Matteucci M, Ravaux JM, et al. Mechanical Circulatory Support as a Bridge to Definitive Treatment in Post-Infarction Ventricular Septal Rupture. JACC Cardiovasc Interv 2021;14:1053-66. [Crossref] [PubMed]
- Estes EH Jr, Dalton FM, Entman ML, et al. The anatomy and blood supply of the papillary muscles of the left ventricle. Am Heart J 1966;71:356-62. [Crossref] [PubMed]
- Panza JA, Ellis AM, Al-Khalidi HR, et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N Engl J Med 2019;381:739-48. [Crossref] [PubMed]
- Noguchi K, Yamaguchi A, Naito K, et al. Short-term and long-term outcomes of postinfarction ventricular septal perforation. Gen Thorac Cardiovasc Surg 2012;60:261-7. [Crossref] [PubMed]
- Coskun KO, Coskun ST, Popov AF, et al. Experiences with surgical treatment of ventricle septal defect as a post infarction complication. J Cardiothorac Surg 2009;4:3. [Crossref] [PubMed]
- Cox FF, Plokker HW, Morshuis WJ, et al. Importance of coronary revascularization for late survival after postinfarction ventricular septal rupture. A reason to perform coronary angiography prior to surgery. Eur Heart J 1996;17:1841-5. [Crossref] [PubMed]
- Pahuja M, Ranka S, Chauhan K, et al. Rupture of Papillary Muscle and Chordae Tendinae Complicating STEMI: A Call for Action. ASAIO J 2021;67:907-16. [PubMed]
- Sulzgruber P, El-Hamid F, Koller L, et al. Long-term outcome and risk prediction in patients suffering acute myocardial infarction complicated by post-infarction cardiac rupture. Int J Cardiol 2017;227:399-403. [Crossref] [PubMed]







