The need for comprehensive multidisciplinary programs, complex interventions, and precision medicine for bicuspid aortic valve disease
Introduction
Bicuspid aortic valve (BAV) is a common congenital lesion with variable manifestations. Most often, BAV leads to the progression of aortic valve stenosis (AS), insufficiency (AI), or aortic aneurysm. On the more severe end of the spectrum, BAV is associated with other congenital heart diagnoses (CHD) such as aortic coarctation or Turner’s syndrome. Rarely, aortic dissection or infective endocarditis can occur. Between 10% and 15% of patients report another family member with BAV, though inheritance patterns and genetic mechanisms remain unclear. Even though most series report a long-term survival similar to that of the general population, BAV patients who present to tertiary and surgical centers may experience higher mortality rates than those in the community (1,2). In short, the heterogeneous and sometimes unpredictable nature of BAV leaves room for improved understanding (3,4).
With the growth of transcatheter valve intervention, multidisciplinary treatment teams have become a guideline-endorsed recommendation for comprehensive and primary valve centers (5). Similar to the heart valve team, we designed a multidisciplinary BAV program to improve clinical coordination and family screening; optimize imaging surveillance and surgery timing; and integrate research efforts that could lead to more precise management of each patient with BAV. In this viewpoint, we review the clinical and research needs of patients with BAV, describing how our program model addresses both. We hope other centers will learn from our program and potentially expand on it with their own subspecialties.
BAV in childhood and adolescence
Patients presenting with BAV in their youth typically demonstrate a more severe manifestation, often associated with other congenital heart disease (CHD) such as aortic coarctation or other left heart obstructive lesions and sometimes with genetic diagnoses such as Turner syndrome (6,7). Fifty percent of pediatric patients with BAV present with associated CHD, versus only 20% of presenting adults (8,9). Even those young BAV patients without associated CHD have been shown to have higher baseline aortic diameter Z-scores than those with BAV and CHD (7), and demonstrate faster aortic growth rates of 0.42 mm/year (10), or change in Z-score of 0.39/year (11).
Aside from presenting with more severe BAV phenotypes, adolescent CHD patients are at risk for attrition as they transition to adult care teams. Male sex, moderate disease severity, changes in insurance coverage, and lack of a formal transition program are all associated with loss to follow up in this age group (12,13). Ideal timing and mode of surgical repair are less established in younger patients, and surgical repair carries increased risk of complications and mortality (14). Additional clinical support and collaboration between adult and pediatric teams is warranted for these patients, who often require surgical or percutaneous intervention earlier in life. As congenital management and prognoses continue to improve, adult cardiac surgery clinics may see a higher proportion of these more complex patients for first-time or re-do surgery.
BAV in adulthood
Patients diagnosed with BAV in adulthood tend to have a more progressive disease course, but heterogeneity exists between their valve morphologies and progression of disease (Figure 1). Among adult BAV patients who present without valvular dysfunction, 24% will require a valve intervention within 20 years of diagnosis, on average 18 years younger than those undergoing surgery for trileaflet AS (16).
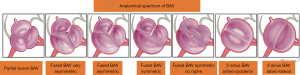
BAV aneurysms affect the aortic root, tubular ascending aorta, aortic arch, or a combination of these three (17). Though there is no universal classification system for BAV aneurysms, several series distinguish ‘root phenotype’ from ‘ascending phenotype’, named according to the region of greatest dilatation. The ascending phenotype, often coupled with AS, is the most commonly seen aortopathy in BAV (18). The root phenotype, associated with AI, is sometimes considered more malignant because it progresses faster than other forms of BAV aortopathy (18,19). Even though BAV aortopathy progresses more rapidly than the general population, the overall risk of dissection remains low (20).
Valvular interventions for BAV
Although surgical criteria are well-defined for AS and AI, the best treatment option is not always straightforward for patients with BAV. Recent valve guidelines organize surgery recommendations based on patient age at time of surgery. Bioprosthetic valve replacement is the recommended choice for patients over age 65; however, because they are prone to structural valve degeneration after 15–20 years, other options are more appropriate for patients under 65, especially those under 50. Shared decision-making becomes especially important for those between 50 and 65 years old (5).
For patients under 50 years old, valve guidelines recommend either mechanical valve replacement, valve repair for AI, or the Ross procedure for AS and select AI at comprehensive valve centers (5). Though mechanical valves require long-term warfarin, patients under 55 years old who received a mechanical valve demonstrated better long-term durability and 15-year survival than those receiving bioprosthetic valves in the same age group (21). More recent versions of mechanical valves offer the same durability within a lower INR range than historically needed (22). For patients who want to avoid anticoagulant therapy because of medical contraindications or lifestyle preference, a valve repair or Ross procedure are excellent alternatives at comprehensive valve centers (23,24).
Severe AS is the most common indication for surgery in patients with BAV, with the majority presenting for surgery between ages of 55–68 years old (25). Surgical aortic valve replacement (SAVR) with a bioprosthetic valve is the gold standard for most BAV patients in this group, who are likely low-risk and/or have a life expectancy greater than 20 years (5). Mini-sternotomy or mini-thoracotomy incisions can be attractive for patients who want a less invasive approach but are ineligible for transcatheter aortic valve replacement (TAVR). A transcatheter valve-in-valve procedure can be performed later in life if the bioprosthetic valve degenerates, so long as it is sized appropriately to host a TAVR valve (5). TAVR is the preferred approach in symptomatic patients with AS over the age of 80, in whom up to 30% have underlying BAV (26), if anatomy is suitable for the transcatheter option.
Considerations for TAVR in BAV
BAV patients made up three percent of those in the STS/ACC registry for low-risk TAVR (27) compared to 18% of low-risk isolated SAVR patients (28). Although experience with BAV TAVR is growing, patients in the TAVR registry represent only a subset of BAV phenotypes with suitable anatomy. Procedural results have improved with newer generation TAVR valves, making TAVR more acceptable for high and intermediate risk BAV patients (29). Anatomic features associated with higher risk of complications and mortality include calcified raphe or bulky leaflets (30). Since patients with BAV stenosis usually need a valve replacement at a younger age than TAV patients, valve size and durability are important considerations for potential intervention on the valve or coronary arteries later in life (31). Patients’ risk factors, life expectancy, and preferences are especially important factors when evaluating their candidacy for TAVR.
Aortic Interventions for BAV
In patients undergoing an aortic valve intervention, prophylactic aortic replacement is recommended for those with aortic diameters 45 mm and above (32). Though less common, some BAV patients need an isolated aortic resection (16); in these cases, surgery is appropriate for aortic diameters 55 mm and above without high risk features. At comprehensive valve centers the threshold can be lowered to 50 mm in the setting of risk factors such as family history of dissection or rapid progression.
Even though aortic diameter and growth rate are the primary markers used to time prophylactic aortic surgery, they are insufficient to predict acute aortic events (33). Institutional factors also influence the timing of aortic intervention; one multi-center series reported that 38% of patients undergoing SAVR and ascending aortic resection had pre-operative aortic diameters below 45 mm (34). This variability in practice may be partly due to differences in perspective about the extent to which genetic and hemodynamic factors contribute to aneurysm progression (35). Research efforts to improve the risk stratification of BAV aortopathy are needed—biomarker development using 4-dimensional flow (4D-flow) cardiac magnetic resonance imaging (CMR) is a promising field (36,37).
The Melman Comprehensive BAV Program at Bluhm Cardiovascular Institute
Although BAV is a common congenital lesion, determining the best management strategy for each patient can be nuanced and sometimes controversial. Referral to subspecialists who have experience with complex interventions, advanced imaging surveillance, and caring for the patient-family with BAV is ideal practice. Collaboration between surgeons, cardiologists, cardiac imaging specialists, and nurses can help improve clinical decision making until more precise biomarkers become available. Equally important in patients requiring valve intervention, patient preference is often the deciding factor for procedure and valve types, underscoring the need for patient education and counsel as they reach an informed decision.
We created a comprehensive BAV program to address the clinical and research needs of patients with BAV (38). Our program includes advanced imaging, complex surgical and transcatheter therapies, family screening coordination, genetic counseling, liaisons between pediatric and adult care teams, a longitudinal clinical database, and translational research initiatives using 4D flow CMR and a growing biobank for BAV and TAV valve and aortic tissues. A designated BAV nurse facilitates the clinical, educational, and research activity between the care team and each patient-family. In addition, we regularly host community events for patients and offer continuing medical education to colleagues.
Patient education initiatives
Patient education at individual and group levels is an important feature of our program. The BAV nurse coordinator provides educational materials and counseling on infective endocarditis prophylaxis (e.g., dental hygiene and antibiotic prophylaxis for patients with prosthetic aortic valves), exercise precautions in patients with aortopathy, family screening (Figure 2), blood pressure control, and post-operative guidelines. Education is chiefly targeted toward patients at critical points in care—those who are newly-diagnosed, preparing for cardiac surgery, and one year post-surgery.
On our BAV Program website, patients can download a file complete with BAV education basics, and/or submit a request to be contacted by the BAV nurse coordinator for more information (39) (Figure 3). On a nearly annual basis, we host in-person or remote events for audiences of 100–200 patients to share updates on new therapies and research developments. Remote events are presented in an online webinar format and recorded to be shared with attendees and future patients alike.

Family history and screening results
In our recent series, 107 (15%) of 887 patients enrolled in our registry reported a family history of BAV; 94 (13%) of these were known at baseline, and another 13 (2%) of patients discovered at least one family member with BAV during follow up. Family screening of 250 first-degree relatives, from 130 families, identified 16 (6%) new first degree relatives with BAV, 11 of whom initiated follow up with our BAV program (38).
For those who are identified with BAV but without valvular dysfunction or aortopathy, patients are seen annually, and we repeat imaging every 3–5 years, provided there are no other risk factors, family history, or changes in physical examination. Transthoracic echocardiography is usually sufficient to measure valvular function (5). To more closely evaluate the aortic sinuses, ascending aorta, and aortic arch, we obtain ECG-gated CT Angiography or CMR Angiography studies for their double-oblique technique and ability to make measurements in consistent locations between studies. Additionally, we found that CMR helps clarify BAV diagnosis and morphology when transthoracic echocardiography results are unclear (40). When planning a long-term imaging strategy for patients with BAV aortopathy, CMR is our preference in order to avoid repeat radiation exposure and to obtain serial 4D flow CMR images.
Adolescent transition clinic
In collaboration with our neighboring Lurie Children’s Hospital, we established a monthly BAV transition clinic for patients over the age of 16 to foster continuity of care. As we recently reported, this clinic follows an enriched population with over 40% of patients reporting mild or greater valve disease, 18% significant aortopathy, and 27% a family history of BAV and/or aortopathy (38). A multidisciplinary team of adult and pediatric clinicians work to optimize the imaging plan, blood pressure control, activity precautions, family screening, and counsel each patient-family about surgery timing. A social worker supports patients with the logistics of transitioning to our adult cardiology clinic, including navigating insurance changes and assessing developmental readiness. Due to common lifestyle changes in this age group—such as relocating to a new city for schooling or work—not all patients are bound to transition to our adult team. Still, this collaboration supports continuity for an at-risk population and has led to referrals for cardiac surgery, as well as BAV screening and follow-up of adult family members.
Procedural volume trends
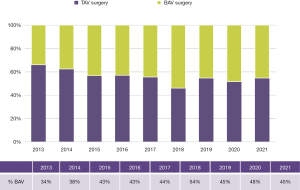
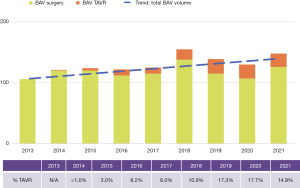
Since 2012 we have completed 1,087 surgical procedures and 110 TAVRs in BAV patients. In addition, over 650 samples of valve or aortic tissue have been collected from patients undergoing surgery. With the growth of TAVR therapy for AS primarily in tricuspid aortic valves, BAV patients now represent a growing proportion of our aortic valve surgery volume (Figure 4). TAVR experience is growing for the treatment of AS in BAV, but is only suitable for a minority of patients. Until randomized trials prove TAVR to be safe and effective for a wider range of BAV patients, it is unlikely that it will become the dominant therapy choice for BAV AS; this is especially true considering how many patients require concomitant aneurysm resection at the time of SAVR (Figure 5).
Clinical database publications
Our BAV Registry now follows over 1,000 patients who are either medically and/or surgically managed. Data is abstracted annually to capture updated imaging results, medications, social history, family history, or new diagnoses. To date, several clinical findings have been published from this registry: we validated the safety of the guideline-based 45 mm threshold for aorta replacement with concomitant valve surgery; demonstrated that CMR is more diagnostic than transthoracic echocardiography for BAV; identified an association between preoperative statin use and lower odds for ascending aortic dilatation; and reported that women with BAV are older, less likely to have AI, and higher operative risk upon surgical referral (32,40-42).
Another recent series describes the first eight years of our program experience, including the characteristics and outcomes of medically and surgically managed patients in our registry (Table 1).
Table 1
| Variable | N | Entire cohort (n=887) | Medical (n=455) | Surgical (n=388) | Medical to surgical (n=44) | P value |
|---|---|---|---|---|---|---|
| Age | 887 | 52.0±14.5 | 46.0±13.6 | 58.9±12.6 | 54.3±11.7 | <0.001 |
| Maximum aortic diameter | 357 | 42.3±5.9 | 41.4±5.1 | 43.9±7.3 | 43.4±4.5 | 0.001 |
| Gender (female) | 887 | 233 (26%) | 156 (34%) | 73 (19%) | 4 (9%) | <0.001 |
| Family history BAV | 704 | 94 (13%) | 64 (14%) | 25 (11%) | 5 (12%) | 0.52 |
| Family history of ascending aortic aneurysm | 859 | 19 (2%) | 16 (4%) | 3 (1%) | 0 (0%) | 0.015 |
| Sievers fusion pattern | 782 | 0.001 | ||||
| Type 0 | ||||||
| Anteroposterior | 27 (3%) | 12 (3%) | 12 (3%) | 3 (8%) | ||
| Lateral | 24 (3%) | 8 (2%) | 14 (4%) | 2 (5%) | ||
| Type 1 | ||||||
| Right: left coronary | 574 (73%) | 308 (77%) | 245 (71%) | 21 (53%) | ||
| Right: non-coronary | 124 (16%) | 61 (15%) | 54 (16%) | 9 (23%) | ||
| Left: non-coronary | 9 (1%) | 6 (2%) | 2 (1%) | 1 (3%) | ||
| Type 2 (unicuspid) | 24 (3%) | 3 (1%) | 17 (5%) | 4 (10%) |
BAV, bicuspid aortic valve.
4D flow MRI findings
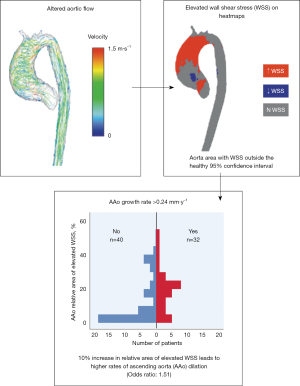
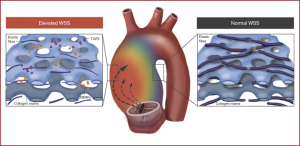
For patients with BAV, 4D flow CMR can measure 3-dimensional hemodynamics and the resulting forces exerted on the aortic wall. Using this imaging technique, we can quantify and locate regions of elevated wall shear stress (WSS) along the aorta of BAV patients. We collaborate with 4D flow CMR specialists in an effort to develop noninvasive biomarkers to improve risk-stratification of BAV aortopathy. Since 2011, we completed 4D flow CMR scans on 1,280 BAV patients and published 51 related manuscripts. In two separate studies we found that elevated WSS is associated with both medial wall degeneration of the aorta and faster rates of progressive ascending aorta dilation (Figures 6,7) (36,37). These studies indicate that the time may arrive when 4-D MRI assessment of WSS becomes an important indicator of aneurysm progression and possible rupture, WSS findings may guide surgical resection instead of the crude anatomic findings of aortic diameter, and postoperative 4D MRI studies may provide information about residual WSS in the remaining aorta.
Conclusions
Bicuspid aortic valve is a common and heterogeneous diagnosis that often leads to valve and aortic intervention. Specialists at comprehensive valve centers can offer a range of complex therapies that suit patients across the spectrum of BAV phenotypes and presentations. Further, a multidisciplinary approach to care should be applied to the treatment of BAV patients and tailored to their unique clinical and translational research needs.
Acknowledgments
Development of the Melman Comprehensive Bicuspid Aortic Valve Program at the Bluhm Cardiovascular Institute was made possible by support from the Martha and Richard Melman family.
Funding: None.
Footnote
Conflicts of Interest: PMM: Royalties: Edwards Lifesciences, Inc.; Speaker fees: Atricure, Inc.; Medtronic, Inc.; Edwards Lifesciences, Inc. SCM: Consultant: Edwards Lifesciences, Inc., Medtronic, Inc.; Cryolife; Terumo Aortic. MM: Research support: Siemens; Grant: Circle Cardiovascular Imaging ROB: Editor-in-Chief, JAMA Cardiology. PWMF: Consultant: Aziyo Biologics Inc., Abyrx Inc. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Michelena HI, Suri RM, Katan O, et al. Sex Differences and Survival in Adults With Bicuspid Aortic Valves: Verification in 3 Contemporary Echocardiographic Cohorts. J Am Heart Assoc 2016;5:e004211. [Crossref] [PubMed]
- Fedak PWM. Bicuspid aortic valve and the specialty clinic: are your patients at risk? Cardiol Young 2017;27:411-2. [Crossref] [PubMed]
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016;151:1650-9.e1. [Crossref] [PubMed]
- Masri A, Svensson LG, Griffin BP, et al. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart 2017;103:1323-30. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72-e227. [PubMed]
- Niaz T, Poterucha JT, Olson TM, et al. Characteristic Morphologies of the Bicuspid Aortic Valve in Patients with Genetic Syndromes. J Am Soc Echocardiogr 2018;31:194-200. [Crossref] [PubMed]
- Niaz T, Poterucha JT, Johnson JN, et al. Incidence, morphology, and progression of bicuspid aortic valve in pediatric and young adult subjects with coexisting congenital heart defects. Congenit Heart Dis 2017;12:261-9. [Crossref] [PubMed]
- Fernandes SM, Sanders SP, Khairy P, et al. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol 2004;44:1648-51. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Holmes KW, Lehmann CU, Dalal D, et al. Progressive dilation of the ascending aorta in children with isolated bicuspid aortic valve. Am J Cardiol 2007;99:978-83. [Crossref] [PubMed]
- Warren AE, Boyd ML, O'Connell C, et al. Dilatation of the ascending aorta in paediatric patients with bicuspid aortic valve: frequency, rate of progression and risk factors. Heart 2006;92:1496-500. [Crossref] [PubMed]
- Madsen CB, Hattersley S, Buck J, et al. Approaches to risk assessment in food allergy: report from a workshop ''developing a framework for assessing the risk from allergenic foods". Food Chem Toxicol 2009;47:480-9. [Crossref] [PubMed]
- Gurvitz M, Valente AM, Broberg C, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol 2013;61:2180-4. [Crossref] [PubMed]
- Karamlou T, Jang K, Williams WG, et al. Outcomes and associated risk factors for aortic valve replacement in 160 children: a competing-risks analysis. Circulation 2005;112:3462-9. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. Speaking a common language: Introduction to a standard terminology for the bicuspid aortic valve and its aortopathy. Prog Cardiovasc Dis 2020;63:419-24. [Crossref] [PubMed]
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776-84. [Crossref] [PubMed]
- Fazel SS, Mallidi HR, Lee RS, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg 2008;135:901-7, 907.e1-2.
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007;31:397-404; discussion 404-5. [Crossref] [PubMed]
- Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging 2013;6:1301-10. [Crossref] [PubMed]
- Detaint D, Michelena HI, Nkomo VT, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014;100:126-34. [Crossref] [PubMed]
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med 2017;377:1847-57. [Crossref] [PubMed]
- Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg 2014;147:1202-1210; discussion 1210-1. [Crossref] [PubMed]
- Buratto E, Shi WY, Wynne R, et al. Improved Survival After the Ross Procedure Compared With Mechanical Aortic Valve Replacement. J Am Coll Cardiol 2018;71:1337-44. [Crossref] [PubMed]
- El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet 2010;376:524-31. [Crossref] [PubMed]
- Yang LT, Tribouilloy C, Masri A, et al. Clinical presentation and outcomes of adults with bicuspid aortic valves: 2020 update. Prog Cardiovasc Dis 2020;63:434-41. [Crossref] [PubMed]
- Roberts WC, Ko JM, Garner WL, et al. Valve structure and survival in octogenarians having aortic valve replacement for aortic stenosis (+/- aortic regurgitation) with versus without coronary artery bypass grafting at a single US medical center (1993 to 2005). Am J Cardiol 2007;100:489-95. [Crossref] [PubMed]
- Makkar RR, Yoon SH, Leon MB, et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA 2019;321:2193-202. [Crossref] [PubMed]
- Ram E, Amunts S, Zuroff E, et al. Outcomes of isolated surgical aortic valve replacement in the era of transcatheter aortic valve implantation. J Card Surg 2020;35:1452-7. [Crossref] [PubMed]
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol 2017;69:2579-89. [Crossref] [PubMed]
- Yoon SH, Kim WK, Dhoble A, et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020;76:1018-30. [Crossref] [PubMed]
- Tarantini G, Fabris T, Cardaioli F, et al. Coronary Access After Transcatheter Aortic Valve Replacement in Patients With Bicuspid Aortic Valve: Lights and Shades. JACC Cardiovasc Interv 2019;12:1190-1. [Crossref] [PubMed]
- Rinewalt D, McCarthy PM, Malaisrie SC, et al. Effect of aortic aneurysm replacement on outcomes after bicuspid aortic valve surgery: validation of contemporary guidelines. J Thorac Cardiovasc Surg 2014;148:2060-9. [Crossref] [PubMed]
- Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J Thorac Cardiovasc Surg 2018;156:e41-74. [Crossref] [PubMed]
- Nissen AP, Truong VTT, Alhafez BA, et al. Surgical repair of bicuspid aortopathy at small diameters: Clinical and institutional factors. J Thorac Cardiovasc Surg 2020;159:2216-26.e2. [Crossref] [PubMed]
- Verma S, Yanagawa B, Kalra S, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: a survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg 2013;146:1033-40.e4. [Crossref] [PubMed]
- Guzzardi DG, Barker AJ, van Ooij P, et al. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights From Wall Shear Stress Mapping. J Am Coll Cardiol 2015;66:892-900. [Crossref] [PubMed]
- Soulat G, Scott MB, Allen BD, et al. Association of Regional Wall Shear Stress and Progressive Ascending Aorta Dilation in Bicuspid Aortic Valve. JACC Cardiovasc Imaging 2022;15:33-42. [Crossref] [PubMed]
- Crawford EE, McCarthy PM, Malaisrie SC, et al. Applications of a Specialty Bicuspid Aortic Valve Program: Clinical Continuity and Translational Collaboration. J Clin Med 2020;9:1354. [Crossref] [PubMed]
-
Northwestern Medicine BCVI, Melman Comprehensive Bicuspid Aortic Valve Program Patient Education Guide 2017 . Available online: https://nm.bav.org - Malaisrie SC, Carr J, Mikati I, et al. Cardiac magnetic resonance imaging is more diagnostic than 2-dimensional echocardiography in determining the presence of bicuspid aortic valve. J Thorac Cardiovasc Surg 2012;144:370-6. [Crossref] [PubMed]
- Taylor AP, Yadlapati A, Andrei AC, et al. Statin Use and Aneurysm Risk in Patients With Bicuspid Aortic Valve Disease. Clin Cardiol 2016;39:41-7. [Crossref] [PubMed]
- Andrei AC, Yadlapati A, Malaisrie SC, et al. Comparison of outcomes and presentation in men-versus-women with bicuspid aortic valves undergoing aortic valve replacement. Am J Cardiol 2015;116:250-5. [Crossref] [PubMed]







