How to implement a clinical robotic mitral valve surgery program
Introduction
The clinical practice of cardiac surgery has changed drastically in recent years as minimally invasive cardiac surgery has come to the foreground with reduced surgical invasiveness, post-operative pain, blood loss, and intensive care/overall hospital length of stay (1-3). Dr. Alain Carpentier performed the first robotic mitral valve repair in May 1998 using an early robotic protype, and in May 2000, Dr. W. Randolph Chitwood performed the first mitral valve repair using the DaVinci system (4). Robot-assisted mitral valve repair is well accepted with excellent results and without compromising patient safety or repair quality (5,6). Studies have also shown that although early direct costs were higher in robotic cardiac surgery, this was balanced by higher late indirect costs in traditional open sternotomy cardiac surgery due to longer intensive care unit (ICU) and overall length of stay, readmission rates, and transfusion requirements (7). However, the process of becoming an expert at utilizing the surgical robot for mitral valve procedures may be intimidating and dissuade some institutions from implementing a robotic mitral valve surgery program (8). While the fundamental factors to implement a new robotic mitral valve surgery program are similar to starting any cardiac surgery program, in order to become proficient with this innovative technology, training a dedicated and committed surgical team and reinforcing the non-technical skills of the individual members of the team is extremely crucial. At our institution, we utilized the DaVinci system (DaVinci Xi surgical system, Intuitive Surgical Inc., Sunnyvale, CA, USA) for all of our mitral valve repair simulation and live cases. Robotic mitral valve surgery techniques have been extensively described elsewhere and will not be described in detail here. The focus instead will be on the most important factors in the successful implementation of a robotic cardiac surgery program.
Institution support
Institutions interested in implementing a robotic mitral valve surgery program should have an established cardiac surgery program with prior experience with minimally invasive mitral valve procedures. Prior to the onset of a robotic mitral valve surgery program, support within the institution is important, including from the hospital CEO, department chair, administrative staff, fellow surgeons, cardiothoracic surgery trainees, anesthesiologists, perfusionists, intensivists, operating room nurses, advanced practice providers, and other ancillary staff. Appropriate infrastructure and active involvement from the team members empower individuals to contribute ideas and feedback to improve outcomes. Sufficient allocation of robotic operating room time is also needed to ensure skills repetition, increasing experience, and progressive increase in case volume to navigate the learning curve. An independent surgical robotic training consulting company that works directly with hospitals to develop successful robotic surgery programs was approved and contracted by our institution to oversee the implementation of our robotic mitral valve surgical program and carry out training simulations.
Robotic module training
The first step to undertake at the onset of a robotic mitral valve surgery program is for team members to be introduced to the robotic system and technology. Representatives from Intuitive Surgical Systems were present onsite to provide detailed education of the robotic system including docking, instrument exchange, and key safety features. Surgeons also complete online training modules and technical skills training on the robotic console simulator. Intuitive surgical systems only provide online modules and onsite training of equipment for cardiac surgery programs and do not offer subsequent clinical training.
Structured team training simulation platform
Mitral valve surgery is one of the most complex and difficult procedures in cardiac surgery due to the complex anatomy of the mitral valve and its diverse pathology. Given this complexity, team training prior to performing robotic mitral valve procedures on patients is critical and essential to ensure successful outcomes. To develop a successful program, it is also important to ensure appropriate knowledge base and experience of team members. Surgeons should be proficient in open cardiac surgery and have expertise in the surgical management of mitral valve disease. However, beyond the surgeon, safe and effective performance in healthcare relies on teamwork as well as nontechnical skills such as communication and leadership (9). Traditionally, training in healthcare has been focused on individual technical and clinical skills. Adoption of formalized team training is increasing in response to the need for educational efficiency, clinical time pressures, and an ethical imperative “not to practice on real patients” (10). High fidelity patient simulators provide a realistic clinical environment with several anatomic and physiologic variations to mimic real clinical problems. Training modules using simulated chest cavities and hearts have been developed for open cardiac procedures but can be applied to robotic cardiac surgery as well (11). We utilized a thoracic cage model and LifeLike BioTissue mitral valve model (LifeLike BioTissue Inc., London, Ontario, Canada) which is made of a polymer processing hydrogel technology that mimics the tissue characteristics of the mitral valve (12). The mitral valve model as shown in Figure 1 may feature posterior leaflet prolapse and any of the biotissue chords can be torn or cut to simulate chordae tendinae repair. Cadaver or animal mitral valve models were not used as they are much more challenging to obtain approval to be brought into the hospital and operating rooms for simulation training due to their biohazard properties.
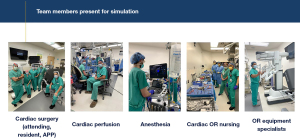
Three of our cardiac surgery advanced practice providers were selected to be designated robotic bedside assistants and were present at the onset of robotic system and simulation training. Although we have a cardiothoracic surgery residency training program at our institution, the involvement of dedicated advanced practice providers was extremely important for consistency as our cardiothoracic surgery trainees often rotate to other clinical rotations. The primary robotic cardiac surgeon at our institution had extensive experience in robotic cardiac surgery, and specifically, in robotic mitral valve surgery. However, for the primary surgeon and other team members to be proficient and efficient in robotic mitral valve surgery, it is essential for a team training simulation platform to be developed. All simulations sessions were performed in the dedicated robotic operating room. The first several team simulation sessions included the surgical team, anesthesiologist, cardiac perfusionists, operating room nursing and equipment specialists to allow for the appropriate configuration of the operating room and set up of the DaVinci robot, robotic consoles, operating table, anesthesia equipment, transesophageal echocardiogram machine, cardiopulmonary bypass machine, and sterile instrument tables (Figure 2).
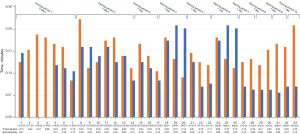
A total of 33 simulation sessions totaling 83 hours of mitral valve repair/valvuloplasty with neochords and annuloplasty with a semirigid band were performed. Critical procedural steps of robotic mitral valve repair were determined which included docking the robot, valvuloplasty, annuloplasty, and atriotomy closure. The duration of each of these critical procedural steps were documented for each simulation session. The three designated advanced practice providers each took turns performing the different procedural steps as bedside assistant. Technical skills were taught and reinforced, as well as the communication required between the bedside assistant and the surgeon at the robotic console.
Through stimulation training, the duration of docking the robot, annuloplasty, valvuloplasty, and atriotomy closure all steadily decreased as team members became more competent, confident, and comfortable in each procedural step and the operation overall. The duration of our simulation training valvuloplasty and annuloplasty are shown in Figure 3. Variability in simulation times were the result of three different advanced practice providers and at times a cardiothoracic surgery resident rotating through different critical steps of the procedure. It may also be due to different experience levels, familiarity with the robot, and affinity to technology. The number of neochords used during valvuloplasty and the number of sutures placed during each annuloplasty simulation also differed, resulting in variable times. Additionally, there were gaps of time between training periods which was reflected in the longer duration times for valvuloplasty and annuloplasty during the first simulation session of the training period. When there were successive simulation sessions, the duration of the critical procedural steps steadily decreased. Overall, the structured team training simulation platform steadily improved the efficiency and flow of critical steps of robot assisted mitral valve repair while enhancing team dynamics.
Debriefing sessions
Following each simulation training day, a team debriefing session was conducted which comprised of all members present at simulation as well as the representative from the independent surgical robotic training company. Each team member was provided the opportunity to reflect on positive aspects of the day’s simulation training as well as room for improvement. The consultant also reviewed and compared the durations of each critical procedure step and offered insight for improvement for the next simulation training session. Additionally, given the different level of progression of the advanced practice providers, the debriefing sessions allowed the surgeon to make necessary adjustments for patient safety and procedural efficiency. If a particular advanced practice provider is not as comfortable with cutting sutures bedside, the surgeon is malleable to compensate for that by the addition of a robotic large cutting scissor to the instrument tray. The debriefing sessions were critically important and contributed to the steady improvement in efficiency and team dynamics throughout simulation training and carried over to the live cases.
Live cases
The number of simulation cases required prior to the first live case is based on comparing duration of critical procedural steps to the “best in class’ benchmark times derived from eight other high-volume institutions who have implemented successful robotic mitral valve surgery programs. Once the surgical team is able to achieve procedural step times at or better than the benchmark times on each critical procedure step then they are able to proceed to a live case. The independent surgical robotic trainer was present for the first 10 live cases after the establishment of our program with in-depth debriefing sessions following each live case.
The structured team training simulation sessions allows surgical teams to perform more complex robotic cardiac procedures at the onset of program establishment. Robotic cardiac surgery procedures are divided into level 1: early learning curve procedures using three robotic arms which include internal thoracic artery takedown, pericardial window, left ventricular lead placement; level 2: intermediate procedures (based on surgeon’s previous minimally invasive surgical experience) which include single vessel robotic minimally invasive direct coronary artery bypass (MIDCAB), multi-vessel robotic MIDCAB, as well as the initiation of four-robotic arm intracardiac procedures. Level 3 advanced procedures include arrest heart totally endoscopic coronary artery bypass graft (AH TECAB), mitral valve repair/replacement, aortic valve replacement, concomitant valve repair/replacement and coronary artery bypass. The recommendation is to start with level 1 robotic procedures and progress to level 3 procedures as experience increases. At our institution, our robotic cardiac surgeon’s extensive prior robotic cardiac surgery experience allowed our program to begin with the intracardiac advanced procedures during simulation as well as the live cases. The structured team training simulation sessions allowed the rest of the team to progress rapidly to be able to assist the surgeon.
Careful patient selection was performed for all live cases. All patients underwent standard preoperative evaluation with the addition of a robotic cardiac surgery protocol chest/abdomen/pelvis computer tomography (CT) to assess the anteroposterior (AP) diameter of the chest, trajectory to the mitral valve annular plane, and femoral vessel anatomy. If the AP diameter is too small, then the heart may be shifted to the left chest and access to the mitral valve becomes difficult. For the initial live cases, the ideal AP diameter to transverse diameter ratio should be close to 1 when selecting patients based on anatomic suitability. Following simulation training, our institution has successfully performed 29 live robot-assisted cardiac surgery procedures with excellent outcomes and no deaths. For the future, we are in the process of developing our institution into a case observation site and center of excellence for advanced robotic cardiac surgery for teams and hospitals across the world to visit for case observation.
Conclusions
Robotic cardiac surgery has drastically changed the clinical practice of cardiac surgery in recent years. Patients benefit from rapid return to presurgical activities, decreased surgical invasiveness, blood loss, and hospital length of stay (1-3). While implementing a robotic mitral valve surgery program and the learning curve may appear daunting initially, developing and executing a structured team training simulation platform to train team members prior to live cases is crucial and allows for a more efficient and safe transition to live cases. If opportunity allows, observing cases at a well-established robotic mitral valve surgery center is also an invaluable experience for team members to gain additional technical and non-technical skills.
The recommendation for establishing a robotic mitral valve program for a surgical team with no prior robotic experience is 20–30 live cases to achieve surgeon autonomy with at least 2 cases per month. This process takes approximately 15–20 months after completing simulated team training. Programs should also aim to perform a minimum of 20 cases per year to maintain proficiency (13).
Lastly, an important factor to also consider in the implementation and maintenance of a robotic mitral valve program is the presence of a referral base that is large enough to maintain adequate case volume to support the program’s experience, quality, and existence. The institution should undergo routine surveillance and evaluation of outcomes to continue to build upon improvements and identify challenges to overcome.
As more cardiac surgeons gain experience with robotic cardiac surgery techniques, the volume of robotic cardiac procedures worldwide will increase to meet patient demand and pave the way for the implementation of new robotic cardiac surgery programs.
Acknowledgments
The authors would like to acknowledge Peter Carnegie from Minimally Invasive Solutions, LLC for his tremendous assistance with developing our institution’s simulation training and guidance during the implementation of our robotic cardiac surgery program.
Funding: None.
Footnote
Conflicts of Interest: BK is a consultant with Medtronic, Boston Scientific, Abbott, Johnson and Johnson. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hawkins RB, Mehaffey JH, Kessel SM, et al. Minimally invasive mitral valve surgery is associated with excellent resource utilization, cost, and outcomes. J Thorac Cardiovasc Surg 2018;156:611-616.e3. [Crossref] [PubMed]
- Gammie JS, Chikwe J, Badhwar V, et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann Thorac Surg 2018;106:716-27. [Crossref] [PubMed]
- Nissen AP, Miller CC 3rd, Thourani VH, et al. Less Invasive Mitral Surgery Versus Conventional Sternotomy Stratified by Mitral Pathology. Ann Thorac Surg 2021;111:819-27. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. A meta-analysis of minimally invasive versus conventional mitral valve repair for patients with degenerative mitral disease. Ann Cardiothorac Surg 2013;2:693-703. [PubMed]
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Güllü AÜ, Senay S, Kocyigit M, et al. An analysis of the learning curve for robotic-assisted mitral valve repair. J Card Surg 2021;36:624-8. [Crossref] [PubMed]
- Blum RH, Raemer DB, Carroll JS, et al. A method for measuring the effectiveness of simulation-based team training for improving communication skills. Anesth Analg 2005;100:1375-80. [Crossref] [PubMed]
- Undre S, Koutantji M, Sevdalis N, et al. Multidisciplinary crisis simulations: the way forward for training surgical teams. World J Surg 2007;31:1843-53. [Crossref] [PubMed]
- Izzat MB, El-Zufari MH, Yim AP. Training model for "beating-heart" coronary artery anastomoses. Ann Thorac Surg 1998;66:580-1. [Crossref] [PubMed]
-
LifeLike BioTissue - Rodriguez E, Nifong LW, Bonatti J, et al. Pathway for surgeons and programs to establish and maintain a successful robot-assisted adult cardiac surgery program. J Thorac Cardiovasc Surg 2016;152:9-13. [Crossref] [PubMed]