Biportal robotic surgery for anterior mediastinal mass
Introduction
Surgical resection remains the standard diagnostic and therapeutic approach for managing anterior mediastinal masses, given the diversity of diagnoses spans from benign cysts to malignancies. Sternotomy is considered a preferred approach because of its easy accessibility to the anatomical loci of lesions (1,2). However, excessive invasiveness and postoperative complications due to open procedures have given way to minimally invasive surgeries, such as video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS). Although VATS has been widely adopted and performed in a myriad of dynamic approaches, including lateral intercostal or subxiphoid multiport approaches, the limited exposure and freedom of movement of thoracoscopic instruments is restrictive for surgeons (3). Robotic-assisted surgery provides an enhanced surgical view of the anterior and upper mediastinum and an increased range of motion, compensating for the limitations of VATS (2). Our institution initiated single-port VATS for anterior mediastinal masses in 2010 and recently reported a case series of minimally invasive surgeries for the robotic single-site-assisted thymectomy through the subxiphoid approach. The disadvantages of the current robotic single-site platform include the non-availability of energy devices and robotic staplers (4). The biportal approach, through the intercostal space (ICS) or subxiphoid, allows the use of these devices with a minimal number of incisions. In this study, we report our surgical techniques of the biportal approach in robot-assisted anterior mass resections and evaluate its safety and feasibility.
Methods
Patients and data collection
This study was a single-center retrospective observational analysis of data collected from electronic medical records. Patients aged >18 years who underwent biportal robotic-assisted anterior mass resection between May 2018 and September 2022 were included. Age, sex, surgical approach (lateral transthoracic or subxiphoid), operative time, conversion to multiport or open procedure, postoperative pathological diagnosis, tumor size, chest drainage duration, length of hospital stay, pain assessment and postoperative complications were reviewed. Patient data of those who underwent a multiport approach for the anterior mediastinal mass surgery were also collected to perform a comparative analysis evaluating the feasibility of the biportal approach. ‘Multiport’ in this paper implies the creation of more than three ports at the beginning of the surgery. Patient selection was made according to the same surgical indications of the biportal-approached cases.
Surgical protocol
The indications for biportal robotic-assisted surgery included an anterior mediastinal mass with or without preoperative pathological confirmation, which requires the use of energy devices and the obtainment of different angles; that is, of a relatively large size (>5 cm), proximity or invasion to nearby vasculatures, lung invasion requiring en bloc resection, or expected diffuse adhesions that require meticulous dissection. In addition, patients who could not be scheduled for a single-port robot-assisted surgery due to the unavailability of the operating theater were rerouted to biportal robotic surgery under informed consent.
After general anesthesia, double-lumen endotracheal tube intubation was performed. This process is required in all cases of the lateral transthoracic approach. In the subxiphoid approach, one-lung ventilation is not mandatory, however; the procedure ensures the availability of one-lung ventilation when en bloc resection of the lung is needed. When two-lung ventilation was used, a small tidal volume (5 mL/kg tidal volume, 15 cycles/min respiration rate, 1:2 inspiratory-to-expiratory ratio without positive end-expiratory pressure) and CO2 insufflation (6–10 mmHg) were maintained by the anesthesiologist (4). The da Vinci Xi Surgical System (Intuitive Surgical, Inc., Mountain View, CA, USA) was used in all the cases.
The lateral transthoracic approach was performed on the right or left side, according to the location of the mass. The patient was placed in a semilateral position, where the ipsilateral chest was slightly elevated by placing a sponge bar under the scapula. A 3–5-cm incision was made in the fifth or sixth ICS on the anterior axillary line. A working port (Lapsingle, Sejong Medical, Paju, South Korea), which consists of four ports and a spring valve for gas circulation, was attached, and CO2 was insufflated. An 8-mm port was inserted in the third or fourth ICS on the anterior axillary line under endoscopic guidance (Figure 1A,1B).
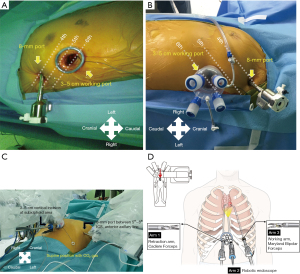
Patients undergoing a subxiphoid biportal approach were placed in the supine position, and the chest at the level of the sternal manubrium was lifted by placing a sponge bar under the back. Port creation in the subxiphoid biportal approach started with a 3–5-cm vertical incision below the xiphoid process. After dividing the linea alba, the retrosternal space was bluntly dissected using a finger. The same working port described for the lateral transthoracic approach was inserted through the incision with CO2 insufflation. Before inserting a second port, the bilateral mediastinal pleura was opened using VATS instruments and a 5-mm endoscope to obtain an adequate operative field. A node grasper (Scanlan International Inc., Saint Paul, MN, USA) and energy devices, such as a harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) or LigaSure (Valleylab, Boulder, CO, USA), were used in this step. After completion of the dissection, an 8-mm port was inserted through the right or left ICS on the anterior axillary line under endoscopic visualization, according to the location of the mass (Figure 1C). The level of insertion can be modified according to the patient’s stature, between the fifth and eight ICS. Figure 1D illustrates the described settings.
In both the lateral transthoracic and subxiphoid approaches, an 8-mm 30° endoscope was introduced through the working port and settled in the center of the robotic arm configuration. Cadiere forceps and Maryland bipolar forceps (Intuitive Surgical, Inc.) were introduced bilaterally to the endoscope, one placed at the farthest of the working port and another set on the second 8-mm port. The switch between forceps and an energy device, such as a vessel sealer (Intuitive Surgical, Inc.) was performed according to need. In cases in which a robotic stapler was needed, the second port was inserted using a 12-mm port with an 8-mm incision. After the specimen was retrieved, one or two drains were placed through the working port incision and the wound was closed. A surgical video of the subxiphoid approach of biportal robot-assisted surgery is provided (Video 1).
Statistical analysis
Categorical variables were expressed as counts and percentages, and Fisher’s exact test was used for comparisons. Continuous variables are expressed as median and interquartile range (IQR). The Mann-Whitney U-test was used to compare the continuous variables. Results were considered statistically significant if the P value was <0.05. IBM SPSS Statistics software (version 23.0; SPSS Inc., Chicago, IL, USA) was used for data analysis.
Results
Clinical characteristics of patients
Table 1 presents a detailed description of the 21 patients who underwent the biportal approach. More than half of the patients were female (n=12, 57.1%), and the median age was 56 years (IQR, 48–61 years). The most common diagnosis was thymoma (n=13, 61.9%), followed by thymic cysts and teratomas (n=3, 14.3% for both diagnoses). The median tumor size was 6.0 cm (IQR, 5.0–6.8 cm). The methods of approach into the thoracic cavity included five right transthoracic, three left transthoracic, and thirteen subxiphoid approaches. There was no conversion to open or multiport surgery, and the median operative time was 165 min (IQR, 140–196 min). Drains were removed at a median of two days (IQR, 1–3 days), and the patients were discharged at a median of four days (IQR, 3–5 days). The median peak pain score assessed using the numeric rating scale (NRS) was 3 points (IQR, 3–5 points). Perioperative complications were reported in two patients. One patient underwent surgical removal of a thymoma with adhesion to the right lung, without invasion. The patient developed postoperative air leak that required multiple sessions of bedside pleurodesis for cessation. After chest drainage removal on postoperative day 9, no recurrence of pneumothorax or pneumomediastinum was observed. Another patient was treated for teratoma, in which the left phrenic and recurrent laryngeal nerves were invaded and resected along with the mass. The patient developed postoperative hoarseness because of unilateral vocal cord palsy. Multiple injection laryngoplasty was performed at the otolaryngology outpatient clinic the following year. There were no cases of readmission or delayed complication.
Table 1
| No. | Sex | Age (years) | Diagnosis | Size (cm) | Approach | Operation time (min) | Drain Removed (days) | Morbidity |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | Thymic cyst | 5.0 | Lt. transthoracic | 190 | 1 | – |
| 2 | F | 56 | Thymic cyst | 4.5 | Rt. transthoracic | 140 | 3 | – |
| 3 | M | 68 | Thymic cyst | 5.5 | Subxiphoid | 146 | 2 | – |
| 4 | F | 51 | Thymoma AB | 6.0 | Rt. transthoracic | 145 | 3 | – |
| 5 | F | 60 | Thymoma AB | 8.5 | Rt. transthoracic | 192 | 2 | – |
| 6 | M | 54 | Thymoma AB | 16 | Subxiphoid | 363 | 9 | Prolonged air leak |
| 7 | M | 65 | Thymoma AB | 6.8 | Subxiphoid | 175 | 1 | – |
| 8 | F | 61 | Thymoma AB | 4.5 | Subxiphoid | 133 | 1 | – |
| 9 | F | 56 | Thymoma AB | 3.3 | Rt. transthoracic | 165 | 1 | – |
| 10 | F | 79 | Thymoma AB | 5.3 | Subxiphoid | 337 | 1 | – |
| 11 | F | 47 | Thymoma AB | 7.5 | Subxiphoid | 160 | 2 | – |
| 12 | M | 42 | Thymoma B1 | 6.6 | Subxiphoid | 220 | 1 | – |
| 13 | M | 48 | Thymoma B1 | 4.1 | Subxiphoid | 132 | 2 | – |
| 14 | F | 63 | Thymoma B1 | 11.9 | Subxiphoid | 316 | 2 | – |
| 15 | M | 39 | Thymoma B1+B2 | 6.2 | Rt. transthoracic | 130 | 3 | – |
| 16 | F | 55 | Thymoma B3 | 6.0 | Lt. transthoracic | 188 | 3 | – |
| 17 | M | 56 | Thymic carcinoma, thymoma B2 | 5.1 | Subxiphoid | 283 | 3 | – |
| 18 | F | 39 | Teratoma | 7.4 | Subxiphoid | 131 | 5 | – |
| 19 | M | 62 | Teratoma | 5.3 | Subxiphoid | 130 | 2 | – |
| 20 | F | 24 | Teratoma | 6.2 | Lt. transthoracic | 155 | 1 | Vocal cord palsy |
| 21 | M | 50 | Bronchogenic cyst | 2.7 | Subxiphoid | 196 | 1 | – |
F, female; M, male.
Comparative analysis with multiport approach
A comparative analysis between the biportal and multiport approaches to robotic-assisted anterior mediastinal mass surgery was performed, and the results are shown in Tables 2,3. There were nine multiport cases in which the majority were lateral transthoracic approaches. Thymoma was the most common diagnosis (n=5; 55.6%). Compared to the multiport approach, the patient population was significantly older (P=0.036). Perioperative outcomes, including conversion rate, operative time, duration of drainage, and hospital stay, showed no significant differences between the two approaches. The postoperative peak pain score assessments of the two groups were also not significantly different.
Table 2
| Characteristics | Biportal approach (n=21) | Multiport approach (n=9) | P value |
|---|---|---|---|
| Sex, n (%) | 0.419 | ||
| Male | 9 (42.9) | 2 (22.2) | |
| Female | 12 (57.1) | 7 (77.8) | |
| Age, median [IQR], years | 56 [48–61] | 42 [37–46] | 0.036 |
| Diagnosis, n (%) | 0.236 | ||
| Thymic hyperplasia | 0 (0) | 1 (11.1) | |
| Thymic cyst | 3 (14.3) | 0 (0) | |
| Thymoma | 13 (61.9) | 5 (55.6) | |
| Thymic carcinoma | 1 (4.8) | 0 (0) | |
| Teratoma | 3 (14.3) | 1 (11.1) | |
| Bronchogenic cyst | 1 (4.8) | 0 (0) | |
| Pericardial cyst | 0 (0) | 1 (11.1) | |
| Atypical carcinoid | 0 (0) | 1 (11.1) | |
| Tumor size, median [IQR], cm | 6.0 [5.0–6.8] | 8.0 [6.4–9.0] | 0.086 |
IQR, interquartile range.
Table 3
| Variables | Biportal approach (n=21) | Multiport approach (n=9) | P value |
|---|---|---|---|
| Approach, n (%) | 0.049 | ||
| Right | 5 (23.8) | 4 (44.4) | |
| Left | 3 (14.3) | 3 (33.3) | |
| Subxiphoid | 13 (61.9) | 1 (11.1) | |
| Bilateral | 0 (0) | 1 (11.1) | |
| Conversion rate, n (%) | 0 (0) | 0 (0) | 1.000 |
| Operative time, median [IQR], min | 165 [140–196] | 215 [196–251] | 0.164 |
| Drainage duration, median [IQR], days | 2 [1–3] | 3 [2–3] | 0.05 |
| Length of stay, median [IQR], days | 4 [3–5] | 5 [4–5] | 0.263 |
| Pain assessment (NRS), median [IQR], points | 3 [3–5] | 3 [3–5] | 0.859 |
IQR, interquartile range; NRS, numerical rating scale.
Discussion
This study reported a biportal approach for robot-assisted anterior mass surgery. The results showed acceptable perioperative outcomes for the conversion rate to open or multiport surgeries, operative time, length of hospital stay, and peak pain scores. Two postoperative complications are reported here: postoperative air leak in a patient with thymoma and lung adhesion where vessel sealer was used for adhesiolysis and vocal cord palsy due to teratoma invasion of the left recurrent laryngeal nerve. There was no significant morbidity or mortality necessitating intensive care.
Many studies have shown superior or equivalent outcomes of minimally invasive mediastinal surgeries in both short- and long-term follow-ups by comparing sternotomy to VATS or RATS and VATS with RATS (1,5-9). Our team has also recently demonstrated the safety and feasibility of robotic single-site-assisted thymectomy (4) for reducing the number of incisions. However, in the current stage of robotic surgical technology, we found that the unavailability of energy devices or staplers on a single-site platform limits the surgical indication to relatively simple cases. Thus, the biportal approach may be used in more complicated cases. Currently, the guidelines suggest sternotomy as the treatment of choice for mediastinal masses larger than 4 cm. However, Alvarado et al. (5) showed in their subgroup analysis comparing the outcomes of VATS and RATS only in large-sized tumor cases (>4 cm), that RATS had a decreased likelihood of having a composite adverse outcome. In our study, many patients with large masses were eventually included because of the indication of the biportal approach surgery (median tumor size was 6 cm). A comparison of the perioperative outcomes with the multiport approach showed that a reduced number of ports showed non-inferior outcomes.
Three approaches using two ports were introduced in this study: the right and left lateral transthoracic and subxiphoid approaches. Most of our cases were thymomas, for which total or total extended thymectomy was indicated. Another limitation of the single-site platform described in our previous article (10) is the inevitable adaptation of VATS during dissection of the lower one-third of the mediastinum. Owing to the innate performance restriction of the system, the site within 8 cm from the port cannot be reached with the robotic instruments. The biportal approach incorporates the da Vinci Xi Surgical System, which has more flexible arms and can overcome this limitation.
Limitations
The first limitation is the small sample size, which limits the generalizability of the results. Compared to other surgical departments, robotic platforms have been adopted relatively recently in general thoracic surgery. In addition, the Korean National Health Insurance System solely pays for reimbursement of all hospital costs but does not cover robotic surgeries. Therefore, many patients are hesitant to pay high costs, which also helps to explain the small sample size. The comparative analysis with the multiport approach cases, which constitutes an even smaller sample size, may have the possibility of type II error. However, had the patient selection in the multiport cases been done under the same criteria as the biportal approach, the error would be minimized. We can only imply that by the zero-conversion rate and comparable outcomes of the drainage duration and length of hospital stay, the biportal approach may be non-inferior to the multiport approach. The small sample size also affected the lack of data on the long-term follow-up, which is the second limitation of this study. The biportal subxiphoid approach is expected to result in the least postoperative pain, although our results show no significant difference compared to the multiport approach. Pain assessment studies should meet our expectations by evaluating long-term follow-up and patients’ quality of life. In addition, the retrospective nature of the study resulted in missing variables, such as the robotic docking time and console time.
Conclusions
In conclusion, biportal robotic-assisted surgery for treating anterior mediastinal masses is feasible and safe. The subcostal approach provides an excellent view of the bilateral phrenic nerves up to the cervical region. This method can offset the drawbacks of the single-site approach, allowing more technically complex surgeries. Further studies with larger sample sizes and long-term follow-ups are needed to better understand the current evolving subject.
Acknowledgments
Funding: This research was supported by Nano·Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (No. 2021M3H4A4079630), the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Ministry of Food and Drug Safety) (project No. 1711138151, KMDF_PR_20200901_0094_02), and the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (No. NRF-2018R1D1A1B07048721).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kang CH, Hwang Y, Lee HJ, et al. Robotic Thymectomy in Anterior Mediastinal Mass: Propensity Score Matching Study With Transsternal Thymectomy. Ann Thorac Surg 2016;102:895-901. [Crossref] [PubMed]
- Radkani P, Joshi D, Barot T, et al. Robotic video-assisted thoracoscopy: minimally invasive approach for management of mediastinal tumors. J Robot Surg 2018;12:75-9. [Crossref] [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Park SY, Han KN, Hong JI, et al. Subxiphoid approach for robotic single-site-assisted thymectomy. Eur J Cardiothorac Surg 2020;58:i34-8. [Crossref] [PubMed]
- Alvarado CE, Worrell SG, Bachman KC, et al. Robotic Approach Has Improved Outcomes for Minimally Invasive Resection of Mediastinal Tumors. Ann Thorac Surg 2022;113:1853-8. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Shen C, Li J, Li J, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for treatment of patients with thymoma: A systematic review and meta-analysis. Thorac Cancer 2022;13:151-61. [Crossref] [PubMed]
- Straughan DM, Fontaine JP, Toloza EM. Robotic-Assisted Videothoracoscopic Mediastinal Surgery. Cancer Control 2015;22:326-30. [Crossref] [PubMed]
- Wu WJ, Zhang FY, Xiao Q, et al. Does robotic-assisted thymectomy have advantages over video-assisted thymectomy in short-term outcomes? A systematic view and meta-analysis. Interact Cardiovasc Thorac Surg 2021;33:385-94. [Crossref] [PubMed]
- Park SY, Kim HK, Jang DS, et al. Initial Experiences With Robotic Single-Site Thoracic Surgery for Mediastinal Masses. Ann Thorac Surg 2019;107:242-7. [Crossref] [PubMed]






