Myocardial protection during minimally invasive mitral valve surgery: strategies and cardioplegic solutions
Introduction
Minimally invasive mitral valve surgery (Mini-MV) has become the preferred surgical approach to treat mitral valve (MV) pathologies, with comparable outcomes to standard median sternotomy (1-3). Mini-MV is more cosmetically attractive to the patient, but more demanding for the cardiac surgeon. Together with improvements in anaesthesia management and postoperative care, surgical outcomes have improved tremendously over the last decade. However, the limited surgical access and increasing complexity of patients (especially reoperations and preoperative heart failure) have focused surgical attention on myocardial preservation strategies.
There is no doubt that adequate myocardial protection plays a key role in achieving successful outcomes in cardiac surgery. However, the optimal cardioplegic solution and cannulation technique for myocardial protection remains controversial. There is little evidence that antegrade or retrograde, intermittent or continuous, crystalloid or blood cardioplegia (with or without additional warm induction and/or hot shot administration), or even beating or fibrillation heart surgery has had any major impact on clinical outcome.
Cardioplegia is not indispensable for myocardial protection (4). Beating or fibrillating heart surgery produces lower levels of postoperative myocardial creatine kinase and troponin T. Beating heart surgery also allows a more physiological assessment of valvular competence and three-dimensional anatomical evaluation of the MV apparatus compared to conventional (arrested) preservation techniques, which might benefit high- risk patients and reoperations (4,5).
For Mini-MV, central aortic crystalloid cardioplegia administration is our standard myocardial protection strategy in patients undergoing primary operations. However, in reoperations where clamping the aorta is not possible due to dense adhesions, porcelain aorta or cardiomyopathy, the right-sided mini-thoracotomy approach can still be safely used by performing the operation on cardiopulmonary bypass (CPB) with a beating or fibrillating heart (6,7) and is our standard alternative to median sternotomy (8). The focus of this article is the Leipzig experience and techniques in relation to the current literature.
Basics of CPB cannulation
For our standard minimally invasive technique (right-lateral mini-thoracotomy), CPB is usually conducted via right femoral-femoral bypass under the guidance of transesophageal echocardiography (TEE) (Figure 1). To minimize hematoma at the planned cannulation site, we recommend that the elective preoperative coronary angiogram be performed on the opposite side. If both the iliac or femoral arteries are atherosclerosed, the aorta is cannulated under direct vision through the lateral thoracotomy, or alternatively the right axillary artery can be exposed and cannulated (Figure 2). In patients who require additional tricuspid valve (TV) or atrial septal defect closure surgery, weigh more than 75 kg, or have inadequate femoral venous return, an additional venous cannula can be inserted percutaneously through the right internal jugular vein and advanced into the superior vena cava, or can be directly cannulated through the mini-thoracotomy.

Standard technique for myocardial preservation
Antegrade crystalloid cardioplegia [Custodial-histidine-trypthophan-ketoglutarate (Custodial-HTK) Brettschneider; Koehler Chemie, Alsbach-Haenlien, Germany] has been used for many years and its safety and efficacy have been established in experimental and clinical studies (9). It is currently the cardioplegia of choice for Mini-MV, as it produces adequate and safe myocardial protection for more than 2 hours after single-dose administration. The Custodial-HTK solution is an intracellular cardioplegic solution developed by Bretschneider in the 1970’s and subsequently modified, containing histidine, acting as a buffer against acidosis during the ischemic period; ketoglutarate, an intermediate of Kreb’s cycle and a precursor of nicotinamide dinucleotide phosphate (NAD) with increasing energy production during reperfusion; tryptophan, functioning as a membrane stabilizer; and mannitol as an antioedematous and free radical scavenger (10,11).
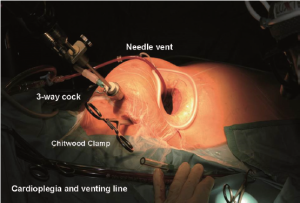
Following implementation of CPB and exposure of the heart, a 7-F cardioplegia needle (CalMed Technologies, CA) is inserted into the ascending aorta at its highest point (Figure 3). It is brought out of the operating field either directly through the small mini-thoracotomy and fixed to a soft tissue retractor (Figure 4) or through a small skin incision through which the MV retractor is inserted. The cardioplegia needle is then connected to a 3-way connector, the other ends of which are connected to the incoming cardioplegia delivery line from the pump and venting line for the aortic root. After antegrade delivery of 1,800 mL of HTK-Custodial cardioplegia at 6-8 °C, it is vented from the aortic root. If the aorta is not completely cross-clamped, blood will also be vented at this time.

Patients undergoing reoperations, or with suspected pleural adhesions, undergo preoperative computed tomography of the chest in order to plan the operative procedure and myocardial protection strategy prior to surgery.
The minimally-invasive approach should be avoided in patients with aortic regurgitation (AR) greater than grade I because of the risks of inadequate cardioplegia delivery and left ventricular distension. Hence, accurate assessment of AR by intraoperative TEE is mandatory to avoid unplanned conversion to a sternotomy during the procedure.
Optimal visualization of the MV apparatus during surgery is essential for successful valve repair. The major advantage of the Custodial-HTK cardioplegia is that an antegrade single shot can maintain myocardial preservation safely for up to two hours, which is normally sufficient to perform even complex MV repairs. By contrast, repetitive administration of blood cardioplegia repeatedly interrupts the surgeon’s work-flow and rhythm. The MV retractor has to be released and removed during cardioplegia delivery to avoid any torsion or incompetence of the aortic valve. In patients with ischemic or non-ischemic cardiomyopathy with poor left ventricular function, we use blood cardioplegia, repeated every 20-30 minutes. However, this requires surgical experience and a stringent work-flow plan to execute the operation efficiently. Hence we do not recommend this cardioplegia technique for all MV repairs and in less experienced hands.
We do not use retrograde cardioplegia during isolated Mini-MV because of the difficulty inserting the retrograde cardioplegia catheter into the coronary sinus (CS). However, in patients undergoing TV procedures, the cannula can be directly inserted into the CS. The exclusive use of retrograde cardioplegia may also provide inadequate right ventricular protection. We therefore use a single shot of cold antegrade Custodial-HTK for primary surgery whenever possible.
Special techniques for myocardial protection
If beating or ventricular fibrillation heart surgery is used instead of aortic cross-clamping, the patient is placed in the Trendelenburg position slightly rotated to the left (to elevate the left atrium relative to the left ventricle) and the aorta vented whenever possible. Fibrillation is induced by either cooling the patient to 28-30 °C or by electrical stimulation. A balloon catheter (e.g., 8F Foley catheter) may be used to vent the left ventricle, especially in patients with mild AR or previous mechanical aortic valve replacement. At the end of the procedure, ventilation is resumed once the left atrium has been completely de-aired, as assessed by TEE.
The Leipzig preservation experience
Our approach for cannulation and myocardial protection for Mini-MV has produced excellent surgical results (2). Our overall observed in-hospital mortality was 1.2%, with a low complication rate compared to conventional surgery. The incidence of severe low cardiac output syndrome following Mini-MV was 1.5%. These patients were managed with inotropic support, intra-aortic balloon pump counterpulsation and/or extracorporeal membrane oxygenation support when necessary. In comparison, Romano et al. observed no cardiac failure after warm beating heart Mini-MV, suggesting better myocardial preservation with this technique (6). The rate of conversion from Mini-MV to midline sternotomy was very low and unrelated to the technique of cardioplegia administration and myocardial preservation (3). Long-term outcomes, reoperation rate, or structural heart disease were not significantly influenced by our myocardial preservation technique (2,8). The overall cumulative survival rate at five years for all patients (including reoperations and heart failure patients) was 87.3% (2).
Over the past decade, we have used this small, muscle-sparing incision and myocardial preservation technique as our procedure of choice in patients requiring reoperations on the MV (12). When the ascending aorta can be safely dissected, it is cross-clamped with a transthoracic clamp with routine delivery of antegrade Custodial-HTK. When the ascending aorta cannot be mobilized, a beating or fibrillating heart technique with full CBP support was employed (the choice of the technique used at the discretion of the surgeon). Similar results with our standardized myocardial preservation algorithm can also be achieved in elderly patients (13) and in those with severely impaired left ventricles (14). Nevertheless, learning, training, mentoring, and re-learning of Mini-MV are essential elements to establish and maintain a professional and successful Mini-MV program (15).
Discussion
Solutions for myocardial protection can be divided by their composition (blood or crystalloids) or according to the temperature at which they are administered (cold or warm). Additionally, they can also be classified as extracellular (with high levels of potassium, magnesium and bicarbonates) or intracellular (low levels of potassium) solutions. At our institution, the Custodial-HTK solution is used as the standard method for potassium-induced electromechanical diastolic myocardial arrest in Mini-MV. There are some potential advantages of Custodial-HTK solution: (I) myocardial protection: the protein buffers such as histidine may be superior to bicarbonate in stabilizing intracellular pH, thus facilitating better recovery of post-ischemic biochemical and mechanical properties (16) and (II) endothelial protection: Yang and colleagues found that Custodial-HTK is superior to the University of Wisconsin solution in protecting endothelium-derived hyperpolarizing factor-mediated endothelium function in small coronary arteries, indicating a good preservation of the vasculature leads to improved functional cardiac recovery (17).
It is known that one single dose of Custodial-HTK may cause hemodilution and hyponatremia if the solution is sucked into the CPB circuit in the routine adult population. Although the serum levels of sodium were generally lower in the postoperative period compared with the preoperative levels in our experience, this had minimal clinical impact as all the levels were within the normal reference range. We also noted that despite low levels of serum sodium, especially during the CBP time, the serum osmolality remained preserved. Different authors speculate that aggressive correction of the transient hyponatremia may, in fact, deleteriously increase the serum osmolality (18,19).
A meta-analysis of 34 trials comparing blood vs. crystalloid cardioplegia failed to show any statistically significant difference between both groups with regard to myocardial infarction or death (20). In this analysis, blood cardioplegia was associated with a lower incidence of postoperative low cardiac output syndrome in 10 of 34 trials, and creatine kinase muscle-brain release was higher in the crystalloid group in 7 of 34 trials. Since all of these studies were on coronary artery bypass surgery, whether these results can be extrapolated to other cardiac operations and specifically to Mini-MV remains unclear. Furthermore, the optimal composition of crystalloid or blood cardioplegia is yet to be established. Braathen et al. recently published a prospective, randomized study comparing single dose Custodial-HTK with repetitive administration of cold blood cardioplegia. The authors concluded that they were equivalent preservation strategies (21).
However, with the growing popularity and acceptance of Mini-MV, many single-centre experiences with different perfusion and protection strategies have been published. The “Leipzig approach” is the most commonly used technique for first-time Mini-MV procedures worldwide. In patients with severe mediastinal adhesions (reoperation, irradiation therapy) or aortic calcification precluding clamping of the ascending aorta, a variety of alternative myocardial protection techniques may be used. In re-do sternotomy cases, Holman and colleagues compared hypothermic cardioplegic arrest, beating and fibrillating heart procedures. They concluded that all three strategies were feasible in most patients and the latter two were at least as safe as surgery performed using cardioplegic arrest (22). They also found that mortality in patients who received cardioplegic arrest was significantly higher than in those who had MV surgery on a beating or perfused fibrillating heart (22).
Recently, Romano and coworkers reported their experience using a beating heart technique for mini-MV (6). The patient body temperature was allowed to drift to approximately 32 °C. Their patients had a shorter bypass time, less transfusion requirements, shorter ventilation time, and lower mortality and stroke rate (6). Beating state with antegrade coronary perfusion avoids reperfusion injury and reduces myocardial edema. This preserves contractile function, which may benefit patients with impaired left ventricular ejection fraction (LVEF) (23). Whether this technique has any impact on perioperative neurological outcomes is unknown. Cerebrovascular accidents (CVA) is particular concern during Mini-MV; the Cleveland Clinic reported a higher stroke rate after right mini-thoracotomy and MV surgery compared to median sternotomy (24). For this reason, care must be taken to ensure complete removal of intracardiac air. We routinely use TEE to assess the mitral and aortic valves and to monitor de-airing. Before releasing the aortic cross-clamp, the patient is placed in the Trendelenburg position, the lungs are ventilated and suction is applied to the aortic root needle vent. Our rate of perioperative CVAs is comparable to that of conventional surgery (2,13,14).
Grossi et al. changed their arterial cannulation technique for Mini-MV from peripheral (femoral vessels) to central (aortic cannulation) because retrograde arterial perfusion was associated with an increased risk of peri-procedural stroke, especially in patients with peripheral vascular disease. Peripheral perfusion should be reserved for selected patients without preoperative evidence of significant atherosclerosis (7,25).
Conclusions
A right anterior mini-thoracotomy with peripheral cannulation is our routine strategy for the majority of patients undergoing MV surgery, including reoperations and patients with cardiomyopathy or porcelain aorta. Myocardial protection is provided by a single dose of antegrade Custodial-HTK cardioplegia, which is an effective and safe technique. Our approach provides adequate myocardial protection, minimal disruption of surgery and excellent long-term results. For reoperations or patients with cardiomyopathy or porcelain aorta, a beating or fibrillating heart technique may be used, which produces comparable results to standard cardioplegic arrest via a midline sternotomy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [PubMed]
- Vollroth M, Seeburger J, Garbade J, et al. Minimally invasive mitral valve surgery is a very safe procedure with very low rates of conversion to full sternotomy. Eur J Cardiothorac Surg 2012;42:e13-5; discusson e16.
- Gersak B, Sutlic Z. Aortic and mitral valve surgery on the beating heart is lowering cardiopulmonary bypass and aortic cross clamp time. Heart Surg Forum 2002;5:182-6. [PubMed]
- Macedo FI, Rodriguez Y, Salerno TA. Myocardial preservation: beating heart techniques. Semin Thorac Cardiovasc Surg 2011;23:314-7. [PubMed]
- Romano MA, Haft JW, Pagani FD, et al. Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: a safe and effective operative alternative. J Thorac Cardiovasc Surg 2012;144:334-9. [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012;143:S68-70. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimally invasive mitral valve surgery after previous sternotomy: experience in 181 patients. Ann Thorac Surg 2009;87:709-14. [PubMed]
- Beyersdorf F, Krause E, Sarai K, et al. Clinical evaluation of hypothermic ventricular fibrillation, multi-dose blood cardioplegia, and single-dose Bretschneider cardioplegia in coronary surgery. Thorac Cardiovasc Surg 1990;38:20-9. [PubMed]
- Bretschneider HJ. Myocardial protection. Thorac Cardiovasc Surg 1980;28:295-302. [PubMed]
- Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res 1992;26:101-8. [PubMed]
- Seeburger J, Falk V, Garbade J, et al. Mitral valve surgical procedures in the elderly. Ann Thorac Surg 2012;94:1999-2003. [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [PubMed]
- Garbade J, Seeburger J, Merk DR, et al. Mitral valve pathology in severely impaired left ventricles can be successfully managed using a right-sided minimally invasive surgical approach. Eur J Cardiothorac Surg 2013;44:e1-7. [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [PubMed]
- Kresh JY, Nastala C, Bianchi PC, et al. The relative buffering power of cardioplegic solutions. J Thorac Cardiovasc Surg 1987;93:309-11. [PubMed]
- Yang Q, Zhang RZ, Yim AP, et al. Histidine-tryptophan-ketoglutarate solution maximally preserves endothelium-derived hyperpolarizing factor-mediated function during heart preservation: comparison with University of Wisconsin solution. J Heart Lung Transplant 2004;23:352-9. [PubMed]
- Lindner G, Zapletal B, Schwarz C, et al. Acute hyponatremia after cardioplegia by histidine-tryptophane-ketoglutarate--a retrospective study. J Cardiothorac Surg 2012;7:52. [PubMed]
- Liu J, Feng Z, Zhao J, et al. The myocardial protection of HTK cardioplegic solution on the long-term ischemic period in pediatric heart surgery. ASAIO J 2008;54:470-3. [PubMed]
- Guru V, Omura J, Alghamdi AA, et al. Is blood superior to crystalloid cardioplegia? A meta-analysis of randomized clinical trials. Circulation 2006;114:I331-8. [PubMed]
- Braathen B, Jeppsson A, Scherstén H, et al. One single dose of histidine-tryptophan-ketoglutarate solution gives equally good myocardial protection in elective mitral valve surgery as repetitive cold blood cardioplegia: a prospective randomized study. J Thorac Cardiovasc Surg 2011;141:995-1001. [PubMed]
- Holman WL, Goldberg SP, Early LJ, et al. Right thoracotomy for mitral reoperation: analysis of technique and outcome. Ann Thorac Surg 2000;70:1970-3. [PubMed]
- Thompson MJ, Behranwala A, Campanella C, et al. Immediate and long-term results of mitral prosthetic replacement using a right thoracotomy beating heart technique. Eur J Cardiothorac Surg 2003;24:47-51; discussion 51. [PubMed]
- Svensson LG, Gillinov AM, Blackstone EH, et al. Does right thoracotomy increase the risk of mitral valve reoperation? J Thorac Cardiovasc Surg 2007;134:677-82. [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Minimally invasive valve surgery with antegrade perfusion strategy is not associated with increased neurologic complications. Ann Thorac Surg 2011;92:1346-9; discussion 1349-50. [PubMed]