Video-atlas on minimally invasive mitral valve surgery—The David Adams technique
Introduction
Mitral valve repair is the gold standard procedure for patients who require surgery for mitral valve disease (1). This is particularly important in the setting of degenerative disease where repair rates of close to 100% are achievable (2). Regardless of the surgical approach (3), minimal access vs. standard open techniques, the chosen therapy should ensure procedural safety and the ability of the surgeon to perform a durable mitral valve repair (4). Herein we demonstrate the less invasive approach we typically use for repair at the Reference Mitral Valve Center at Mount Sinai, the hallmark of which is a limited 7-8 cm lower midline incision, tunneling, full sternotomy, and standard cardiopulmonary bypass techniques with central cannulation.
Technique
The patient is placed in a recumbent position with both arms tucked at each side. Prior to skin prep, a straight line is marked from the sternal notch to the xyphoid. The lower border of the ribs is marked bilaterally and the lower border of the breast is also delineated in females (Figure 1A). The skin is next prepped and draped from the neck to the knees allowing exposure of subclavian and femoral vessels in the event that access is needed. A 7-8 cm incision is made in the lower portion of the chest and the incision is carried down to the sternum. The fascia overlying the pectoralis muscle is freed providing more laxity to the skin and the subcutaneous tissue for better retraction and exposure. The insertion of the pectoralis muscle to the sternum is left intact. Once visualization of the sternal notch is accomplished, the midline of the manubrium and the sternum can be identified and marked with Bovie cautery for subsequent sternotomy. In the absence of xyphoid calcification, this is split in half with Bovie cautery to allow insertion of the sternal saw.
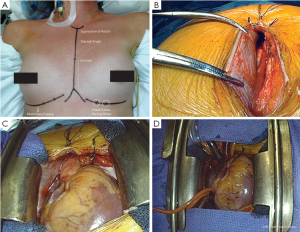
Once the skin and subcutaneous tissue have been mobilized and the midline identified and marked from the xyphoid to the sternal notch, a reciprocating saw is used to divide the sternum from the xyphoid upwards as far as the skin incision allows. An Army-Navy retractor is then placed in the divided portion of the sternum and turned 90 degrees to spread the two edges of bone and facilitate the division of the remaining sternum and manubrium with the oscillating Precision® saw (Stryker Instruments Kalamazoo, MI). Hemostasis of the sternum is then obtained with Bovie cautery and bone wax. An extension to the Bovie can be attached to reach the upper manubrium and hemostasis can be obtained for the crossing veins in the region of the suprasternal notch. These can sometimes become a nuisance as the distance makes it difficult to place sutures or clips. In this scenario, hemostasis of the suprasternal notch can be accomplished after placing a retractor.
Once hemostasis is achieved, a modified (we customize the blade size in a machine shop) or pediatric Cosgrove® retractor (Kapp Surgical Instruments Cleveland, OH) is used to separate the sternum and allow access to the mediastinum (Figure 1). We shorten the retractor blades to facilitate the use of smaller incisions while still using the same soft-tissue atrial retractors that attach to the edges of the blades (Figure 1C). The thymic tissue is then lifted off the pericardium and spared, which minimizes soft tissue dissection leading to less scarring and potential venous bleeding. The pericardial sac is then opened using the aorta as the midline. Division is carried superiorly to the pericardial reflection on the ascending aorta leaving 2-3 mm of pericardium that can be used by pericardial stay sutures to lift the aorta towards the incision. The division of the pericardial sac is then completed inferiorly in a standard fashion. The pericardial edge on the right side is then grasped with two soft-tissue clamps (Figure 1B), the retractor is then removed and then re-applied, sandwiching the pericardium between the retractor blade and the right sternal edge (Figure 1C). The clamps allow the surgeon to pull the pericardium while the retractor is opened, which further lifts the right atrium and cava towards the skin edge improving exposure and minimizing the distance between the skin edge and the heart. Once this is performed, the aorta is scanned with epiaortic ultrasound prior to cannulation. Heparin is then administered and cannulation sutures can be placed on the aorta. A clamp can be used to retract the aorta inferiorly into the field, and the skin in the upper border of the incision can be pulled superiorly with a hand-held retractor to expose the distal portion of the ascending aorta for cannulation. If the distance is too great, or the angle of exposure is too difficult, a Fem-Flex (Edwards Life sciences LLC) cannula is placed in the ascending aorta using a Seldinger technique. Cannulation of the vena cava is facilitated with the use of smaller cannulas, size 24 French, and we typically use vacuum assistance. Retrograde cardioplegia is used routinely on all mitral procedures via standard coronary sinus cannulation.
Once cannulas are placed (Figure 1D), and prior to the institution of cardiopulmonary bypass (CPB), Soondergard’s groove is developed by carefully dissecting the inter-atrial space to the level of the septum. This provides better exposure of the valve by shortening the distance from the atriotomy to the mitral valve. Once dissection is complete, CPB is instituted. A standard antegrade is then established in the ascending aorta with a side-port for root suction. We do not routinely use caval tapes as this further crowds the field and is not required with vacuum assistance. The cross-clamp is applied and antegrade cold blood cardioplegia is infused for a 5-minute period, followed by cold blood retrograde cardioplegia given every 20 minutes for approximately four minutes. An insulation pad is placed along the inferior wall of the heart and topical slush is placed over the right ventricle. The field is also flooded with carbon dioxide.
Excellent exposure of the mitral valve is achieved via a curvilinear incision extended inferiorly to the midpoint between right inferior pulmonary vein and the inferior vena cava. If further exposure of the left atrium is required, the pericardial reflection on both vena cava is released and blunt dissection is used to free the lateral aspects of both veins for about 2 to 3 cm. In addition, the left pericardial reflection can be released fully opening the left pleural space. The mitral procedure (Figure 2A-C) can then be performed as demonstrated in the video, with ample exposure of the valve to enable employment of the same mitral repair techniques available through any other incision including a standard size (12-18 cm) sternotomy. There is also sufficient exposure for tricuspid and aortic valve repair or replacement if needed. Once the procedure is completed, weaning from CPB and decannulation is standard. The protamine is then administered and once hemostasis is assured, the sternum is closed with wires in a standard fashion. Once the wires are placed, the subcutaneous tissue is closed, tacking the fascia to the periosteum of the sternum and manubrium in order to obliterate space created by the tunneling at the beginning of the procedure. If the dissection or tunneling was particularly lengthy it may be difficult to completely close the space between the soft tissues and the sternum. In this setting, in order to avoid the potential formation of a seroma, two techniques can be performed: (I) interrupted circular sutures can be placed in order to tack the subcutaneous tissues to the pectoralis muscle and close the space; and/or (II) a small Jackson-Pratt drain might be left. Typically, this drain remains in place for 3-4 days to ensure fluid removal and can be removed once the daily output is below 50 cc of fluid.
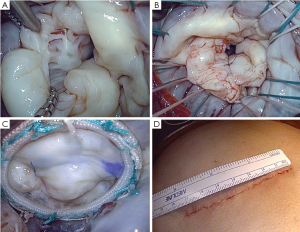
Closure of the skin often requires the circumferential removal of 1 mm of skin from the wound, as retraction often devitalizes the skin and may lead to superficial dehiscence and a larger scar. The skin can then be closed with standard absorbable sutures (Figure 2D). The postoperative care is no different than any standard-sized open sternotomy approach.
Comments
A variety of less invasive techniques have been described to limit the trauma and morbidity associated with a “standard sternotomy” approach in mitral valve surgery (5-7). Although this incision maximizes exposure for the surgeon, it can be painful for the patient, and is associated with increased risks of bleeding and less “scar satisfaction” compared to less invasive approaches. Many excellent surgeons and centers have perfected various approaches both from the midline and via a right thoracotomy to avoid a “standard sternotomy” with excellent results in terms of patient satisfaction and quality mitral valve repair. Dr. Cosgrove at Cleveland Clinic (8) and Dr. Lawrence Cohn at Brigham and Women’s Hospital (9), among others pioneered efforts to perform less invasive mitral surgery through limited midline approaches, usually employing partial sternotomy. Our group initially followed their experience; however, we later evolved to complete sternotomy with myocutaneous flaps, as we found often times the sternal closure was easier after complete sternotomy. We have found this technique to achieve all of the goals of less invasive surgery: (I) excellent cosmesis and very high patient satisfaction; (II) minimizing trauma with excellent post-operative pain control (most of our patients do not utilize narcotics after leaving the hospital); (III) low rates of bleeding and transfusion (our re-exploration rate for bleeding is <1%); and (IV) the ability to perform any reconstructive technique that would be used in a standard sternotomy, with very high repair rates (our most recent series documented a repair rate exceeding 99% in an all-comers population of degenerative disease regardless of complexity). Although the limited incision we use adds complexity and certainly operative time compared to a standard sternotomy, it can be mastered by all experienced surgeons and does not require specialized equipment other than shortened blades on a mitral retractor.
Acknowledgements
Dr. Adams is an inventor and consultant for Edwards Lifesciences and Medtronic.
Disclosure: The authors declare no conflict of interest.
References
- David TE, Armstrong S, McCrindle BW, et al. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013;127:1485-92. [PubMed]
- Castillo JG, Anyanwu AC, Fuster V, et al. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg 2012;144:308-12. [PubMed]
- Seeburger J, Borger MA, Doll N, et al. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur J Cardiothorac Surg 2009;36:532-8. [PubMed]
- Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609-16. [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003;108 Suppl 1:II48-54. [PubMed]
- Chitwood WR Jr, Rodriguez E, Chu MW, et al. Robotic mitral valve repairs in 300 patients: a single-center experience. J Thorac Cardiovasc Surg 2008;136:436-41. [PubMed]
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [PubMed]
- Cosgrove DM 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg 1998;65:1535-8; discussion 1538-9. [PubMed]
- McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [PubMed]





