TEVAR for complicated acute type B dissection with malperfusion
Clinical scenario
A patient presents with an acute type B aortic dissection (ABAD) and malperfusion is in his fifth or sixth decade of life, with a history of hypertension and an acute moment of chest pain and left leg pain (Video 1). He then develops associated abdominal pain and persistent left leg pain with diminishing sensation and movement. This type of patient requires urgent intervention, with the currently preferred modality of treatment being thoracic endovascular aortic repair (TEVAR) (1). TEVAR allows restoration of end-organ perfusion and may allow for favorable reverse remodeling to heal the aorta.
Surgical techniques
Preparation
The patient is placed in a supine position under general anesthesia with mechanical ventilation, and a lumbar drain is placed in the selected patient following induction of general anesthesia. Antibiotics are administered prior to the procedure and the patient is prepped and draped in a standard fashion.
Exposition
Stent graft delivery is usually accomplished through a transfemoral approach, involving a standard transverse infrainguinal incision to expose the common femoral artery. Percutaneous access is attained through the contralateral femoral artery to advance a marker pigtail catheter for angiography.
Operation
Intravascular ultrasound (IVUS) is critical to ensure that the working wire and stent graft will lie within the true lumen. The stent graft is positioned under fluoroscopic guidance and IVUS is used for optimal guidance. Following deployment, the stent graft location is evaluated using aortography and IVUS, and if type I and/or type III endoleaks are revealed, they are instantly treated. Balloon dilation of the overlap zones is performed if multiple stent grafts are deployed. Branch vessel investigation is conducted using arteriography and branch vessel pressure manometry. If residual malperfusion is identified due to static obstruction, branch vessel stenting with self-expanding stents is performed.
Completion
All catheters and wires are withdrawn, followed by primary repair of the femoral artery using interrupted 5-0 Prolene. Doppler signals are checked and confirmed. 2-0 Vicryl is used for primary closure of the deep and superficial fascia, and 4-0 Monocryl is used for subcutaneous closure of the skin. This is followed by administration of protamine to neutralize heparin.
Comments
Clinical results
Of the 454 patients presenting with ABAD in our practice at the University of Michigan Health System from 1995-2012, 49 patients underwent TEVAR, of whom seven suffered malperfusion. The in-hospital mortality in this group of patients was 14.3% (1 of 7) (2). A significant number of the patients during this time period were treated with percutaneous flap fenestration and true lumen stenting as an alternative to open repair or TEVAR. However, with the acceptance of TEVAR as a primary treatment option, our strategy has evolved towards this type of management (3,4).
Advantages
Preoperative workup for TEVAR with malperfusion should include dynamic computed tomography (CT) imaging from the thoracic inlet to the femoral arteries. This imaging modality can give information on whether branch vessel obstruction is present and by which mechanism, and also if an associated aortic aneurysm exists. Preoperative planning with 3D reconstruction of CT scans is even more important for the durability of TEVAR, using adequate sizing and assisting in determining suitability of TEVAR itself. Not only does the affected segment and landing zones need to be analyzed, but the access vessels can also be assessed during the preoperative imaging workup.
In our practice, left subclavian revascularization is only performed selectively and concomitantly in patients with a dominant left vertebral artery, left internal mammary artery (LIMA) to left anterior descending (LAD) coronary artery graft, or a left vertebral artery directly arising from the aortic arch.
The stent graft is deployed to cover the primary entry tear within the true lumen. Subsequently, the true lumen expands, and dynamic branch vessel compromise is ameliorated. By eliminating flow through the primary entry tear, false lumen thrombosis is induced due to significantly reduced false lumen flow. If static branch vessel obstruction occurs at this point, it is treated with self-expanding stents. This approach may treat both the malperfusion component and the initial pathologic aortic condition. In contrast, percutaneous fenestration and true lumen stenting only treats the malperfusion component.
The different pre- and intraoperative steps of TEVAR for patients with ABAD, complicated by malperfusion, are further illustrated in Figures 1-5.
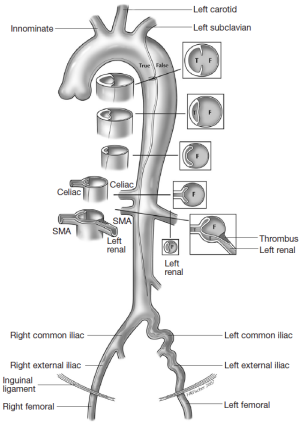
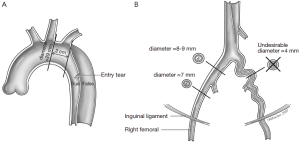
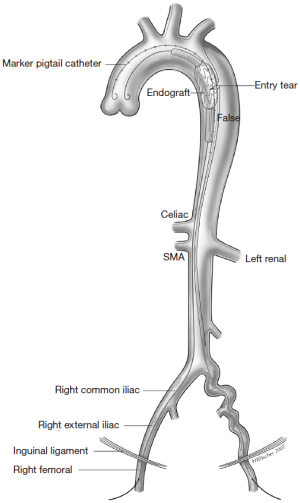

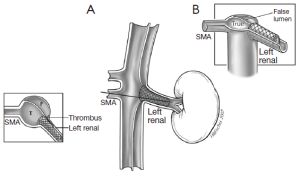
Caveats
At times, branch vessel obstruction from dynamic malperfusion may be suspected on preoperative imaging and clinical findings. When performing the operation, a lack of malperfusion of selected branch vessels may be identified. The etiology of this could include the following. Firstly, the patient may have sustained multiple re-entry tears from the time of the initial imaging study to the time of operation, which could spontaneously resolve the malperfusion syndrome. Secondly, the initial imaging study may have had ill-timed contrast boluses, suggesting a lack of blood flow to viscera. This would not have been apparent if phase delay imaging had been performed.
Acknowledgements
Dr. HJ Patel and Dr. DM Williams have consulting relationship and patent with WL Gore.
Disclosure: The authors declare no conflict of interest.
References
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [PubMed]
- Wilkinson DA, Patel HJ, Williams DM, et al. Early open and endovascular thoracic aortic repair for complicated type B aortic dissection. Ann Thorac Surg 2013;96:23-30; discussion 230. [PubMed]
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [PubMed]
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340:1539-45. [PubMed]
- Patel HJ, Williams DM. Endovascular therapy for malperfusion in acute type B aortic dissection. Operative techniques in Thoracic and Cardiovascular Surgery 2009;14:2-11.





