Open repair of chronic complicated type B aortic dissection using the open distal technique
Introduction
In 2008, the Society of Thoracic Surgeons convened a task force to evaluate the current data at the time regarding thoracic endovascular aortic repair (TEVAR) for descending thoracic aortic pathologies. From this, the panel’s consensus document recommended that the management of chronic type B aortic dissection should remain medical unless complications of enlargement, redissection, malperfusion, or refractory symptoms ensued (1). Furthermore, the consensus document went on to suggest that “patients with chronic aortic dissection should always be considered susceptible to the late sequelae of the disease regardless of therapy chosen during the acute phase”. Although this document did not formally address the management of chronic aortic dissection, it stated that “both open surgical and endovascular stent-graft treatment may slow the disease, but neither reverses its natural history unless the entire extent of dissection is either resected or excluded, and that can be achieved only by surgical intervention”.
Since then, a number of series reporting the use of TEVAR for complicated chronic type B aortic dissection have been published, touting improved early results when compared to open repair (2-8). Recently published guidelines from a European Interdisciplinary task force suggested that patients with chronic type B aortic dissection who develop complications may be treated with endovascular intervention if anatomically suitable. Otherwise, open surgical repair should be utilized for patients who are unable to undergo endovascular repair (9). This suggests that TEVAR should be performed as a first line treatment for complicated chronic descending thoracic aortic dissection (CDTAD). These recommendations were made based on improved early results when compared to open surgical repair in complicated dissection. Although medical management for uncomplicated CDTAD remains the mainstay, recent studies have recommended a change in standard approaches to thoracic endovascular aortic repair (TEVAR) in the acute setting, even when uncomplicated (10,11). However, few studies reporting long-term results from open repair of CDTAD exist (12-14). Thus, the aim of this study is to report early and late outcomes after open repair of CDTAD.
Methods and materials
The Committee for Protection of Human Subjects and the local institutional review board approved this study. Between 1991 and 2013, open repairs of the descending thoracic aorta were retrospectively identified from the departmental database. Indications for intervention included aneurysmal enlargement >5 cm, rapid enlargement (>5 mm per year), and fistulization (bronchial or esophageal). Descending thoracic aortic aneurysms (DTAAs) were classified and distributed according to Figure 1 (15).
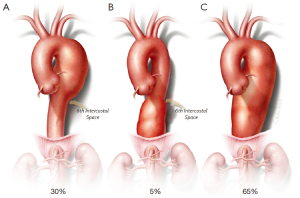
We considered aneurysms with dissection acute if surgery was performed in less than 14 days from the onset of pain, and chronic if after 14 days. Acute dissection was not included in this analysis. Operative mortality included in-hospital death and death occurring within 30 days of surgery. Postoperative immediate neurologic deficit (IND) was defined as paraplegia or paraparesis observed upon the patient awakening from anesthesia, regardless of severity. Delayed neurological deficit was defined as new paraplegia or paraparesis occurring postoperatively in a previously neurologically intact patient. Patients with strokes were identified by a thorough neurologic examination and computerized tomography (CT) or magnetic resonance imaging (MRI) of the head, and were excluded from the neurologic deficit group; hence, ‘neurologic deficit’ referred to paraplegia and paraparesis only. Reintervention pertained to both involved and uninvolved aortic segments. An uninvolved segment referred to any aortic segment other than the descending thoracic segment repaired at the index operation. The glomerular filtration rate (GFR) was calculated by the Cockcroft-Gault method and definitions of other variables were described in previous reports (15,16).
Surgical approach
After general anesthesia, the patient was positioned in the right lateral decubitus position and a cerebrospinal fluid catheter was placed, to allow cerebrospinal fluid drainage and monitoring of CSF pressure. A neurophysiologist monitored somatosensory and motor-evoked potentials. After exposure of the descending thoracic aorta, the patient was administered 0.5-1 mg/kg body weight of sodium heparin, maintaining an activated clotting time between 180 and 250 seconds.
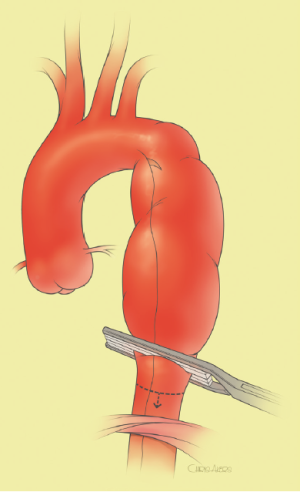
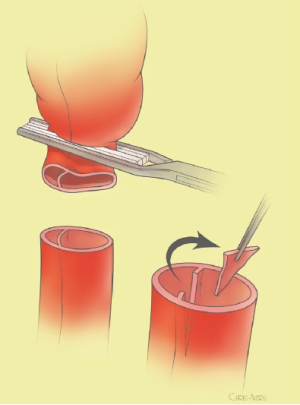
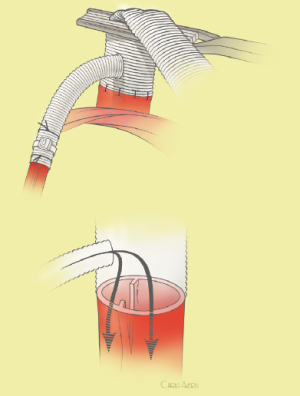
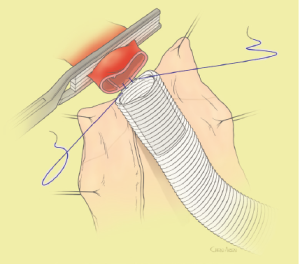
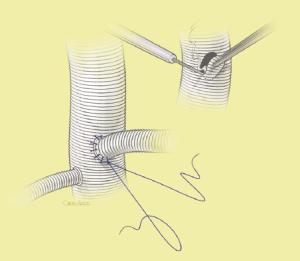
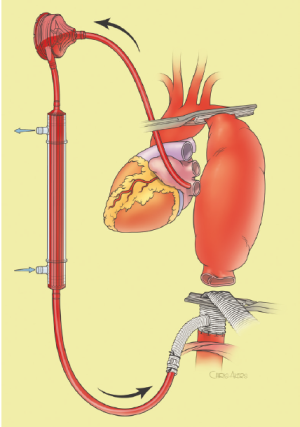
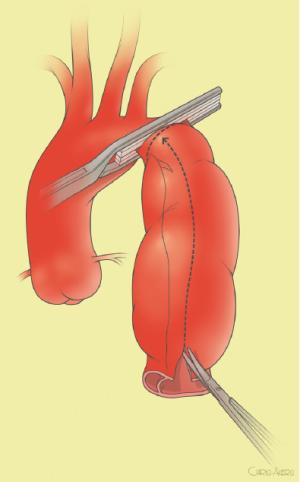
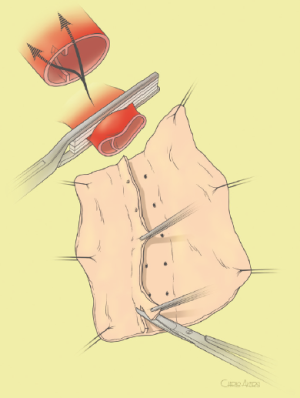
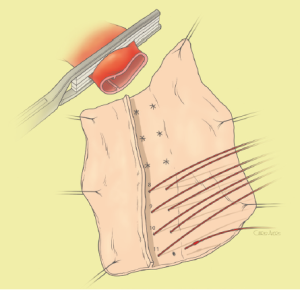
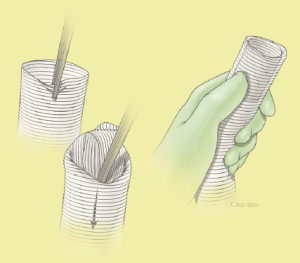
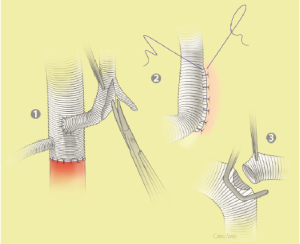
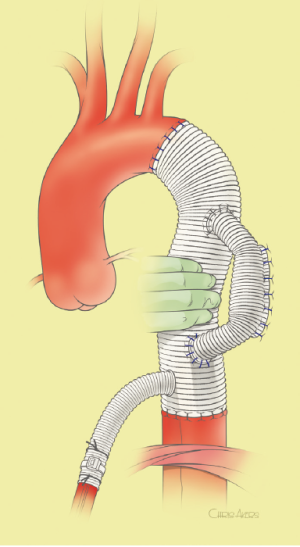
The surgical technique will be described for the “distal first approach”. A second side branch (14 mm Dacron graft) was attached to a commercially available single armed 10-mm Dacron graft which would be used for arterial in-flow for distal aortic perfusion. The newly reconstructed 14 mm side arm would be used for intercostal artery reattachment in the case that this was indicated (Figure 2). The left inferior pulmonary vein was cannulated, to which a BioMedicus (Minneapolis, MN) pump with an in-line heat exchanger was attached. The arterial inflow was established through an 8-mm Dacron graft via the descending thoracic aorta, once the distal anastomosis had been completed (Figure 3). Depending on the extent of the aneurysm, a distal aortic clamp is applied at the level of Thoracic level 8 (T8) to T10 (Figure 4). The distal aorta was opened and completely transected. The dissection membrane (flap) was identified and a wedge-shaped section was resected to allow flow to both the true and false lumen distally (fenestration) (Figure 5). The distal anastomosis was then performed and established, typically using a 3-0 running polyprolene suture. After completion, distal aortic perfusion was initiated via the side arch graft (Figure 6), An aortic clamp was then placed proximally, either proximal or distal to the left subclavian artery (Figure 7). Subsequently, the remaining descending thoracic aorta was opened (Figure 8). Patent intercostal arteries were controlled either by suture ligation for T3 to T8, or balloon catheter occluded using #3 Fogarty catheters (Figure 9). A reversed elephant trunk (Figure 10) was proximally created and the proximal anastomosis was performed (Figure 11). Once completed the aortic clamps were removed, distal pulsatile flow was reestablished. The decision to re-implant patent, lower intercostal arteries (T8-T12) was guided by neuromonitoring (17). If reattachment of the lower intercostal arteries was required, a Loop graft was created for reimplantation (Figure 12,13).
Statistical methods
Data were collected from chart reviews performed by a trained nurse evaluator and entered into a dedicated Microsoft Access database. Analysis was retrospective. Patient follow-up was obtained by direct patient contact, telephone interview or via the National Death Index. Data were managed under HIPAA confidentiality guidelines in a Microsoft Access database with encrypted patient identifiers. Dichotomous bivariate measures of association were computed by contingency table methods with odds ratios and test-based 95% confidence intervals. Multivariable assessment of 30-day mortality risk factors was conducted using multiple logistic regression analysis. Multivariable risk factor assessments for long-term survival were conducted by multiple proportional-hazards regression. Separate long-term probabilities of redo operation and progression of disease into previously uninvolved aorta were estimated using univariate Kaplan-Meier analysis. Comparative survival for the reference population was estimated by applying age-specific annualized US population survival rates to the starting value of the patient cohort at postoperative year one. All computations were performed using SAS software, version 9.3.
Results
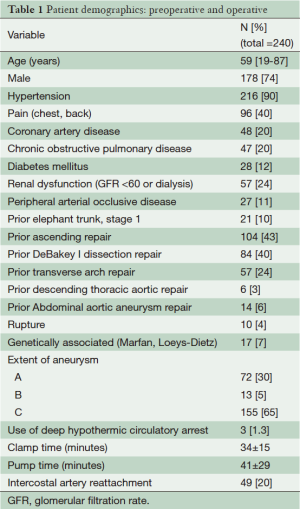
Between 1991 and 2013, 240 (40%) descending thoracic aortic repairs with associated chronic dissection were performed. Patient characteristics are listed in Table 1, with a mean age of 59 years, and 178 (74%) men. The majority of patients (218, 91%) underwent repair using the adjunct of distal aortic perfusion with cerebral spinal fluid drainage.
Full table
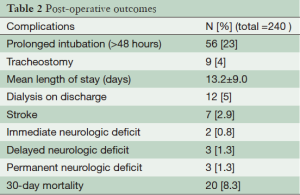
For patients undergoing open repair of CDTAD, the early mortality was 8.3% (20/240). IND occurred in two patients (0.8%), all in the extent C resection. Delayed neurologic deficit (DND) occurred in 4 (1.7%). Since 75% (3/4) of DND patients recovered function, permanent neurologic deficit occurred in 3 (1.4%). Stroke occurred in 7 (2.9%), acute renal failure requiring dialysis on discharge in 12 (5%). Other early outcomes are listed in Table 2.
Full table
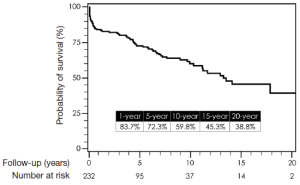
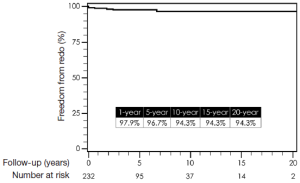
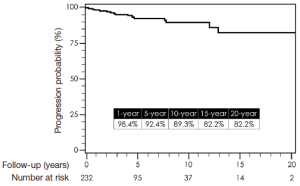
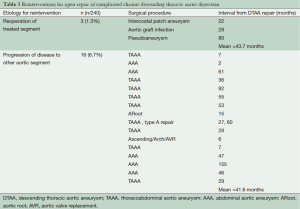
Total follow-up time was 22.6 years and was complete for mortality. Median follow-up time was 8.5 years. One-, 5-, 10-, 15-, and 20-year survival by Kaplan-Meier estimates were 84%, 72%, 60%, 45%, and 39%, respectively (Figure 14). Freedom from reoperation on the operated segment (i.e., descending thoracic aorta) were 97%, 94%, 94% and 94% at 5, 10, 15 and 20 years, respectively (Figure 15). Freedom from reoperation of an uninvolved aortic segment, i.e., progression of aortic disease in another location were 92%, 89%, 82% and 82% at 5, 10, 15 and 20 years, respectively (Figure 16). The types and causes of reintervention are listed in Table 3.
Full table
Comments
Controversy on the best management for complicated CDTAD still remains. Understandably, endovascular therapy is becoming the primary treatment for many diseases of the thoracic aorta due to its less invasive nature and potentially improved early outcomes. With this in mind, many have adopted the use of endovascular treatment for CDTAD, despite the fact that there are only few reports of mid-term results and no long-term data regarding TEVAR for CDTAD exist (6,8). In view of this, we have reported late outcomes for open repair for CDTAD. Although late survival remains consistent with other previous reports on open repair of thoracic aortic disease, this report also demonstrates the durability of open repair of the diseased descending thoracic segment (7,14). Essentially, once open resection and graft replacement has been performed, that segment of the aorta will be free from reintervention in 94% of cases at almost 20 years (Figure 15). This was not unexpected, since some have argued that the only definite approach that eliminates recurrent aortic disease is to resect that involved segment of aorta (1,18). Moreover, as depicted in Figure 16, the late risk for intervention on an uninvolved aortic segment, e.g., progression of disease, was relatively small with a 20-year freedom from progression of disease requiring intervention in 82%. This would suggest that the remaining thoracoabdominal aorta in patients who undergo repair for DTAA after dissection remains stable. However, this interpretation must be made with caution, since other patients who developed more extensive thoracoabdominal aneurysmal disease from chronic dissection were not included in this analysis. Mid- and long-term results from TEVAR for complicated CDTAD will be required so that equivalent comparisons to open repair can be made.
Many have settled on the classification of aortic dissection according to the Debakey of Stanford classification schemes (19,20). More recent detailed classification schemes have been proposed but still remain laborious, thus limiting universal acceptance (21). For this study, we described the specific anatomical segment resected, the descending thoracic aorta, and avoided the use of either the DeBakey or the Stanford classification schemes. The Stanford classification, although therapeutically practical, was too simplistic as it did not define the anatomical involvement in enough detail, and this becomes critical in an era of endovascular therapies. DTAAs could develop as a late complication of a repaired or unrepaired type A dissection or from de novo type B dissection, and the Stanford system was limited as it did not specify whether only the descending thoracic aorta or the entire thoracoabdominal aorta and iliac vessels were involved. The DeBakey classification is a more anatomically detailed description of the dissection, allowing for differentiation between those that involve both the ascending and descending or thoracoabdominal aorta such as with DeBakey Type IIIb dissection. However, this classification fails to delineate a dissection that initiates within and involves the transverse arch. At any rate, an accurate description of a dissection would entail that which was proposed by Dake et al. (21), but by convention, the original scheme by DeBakey has become more pertinent in the current era.
Although this series occurred over 20 years, the fundamental techniques used for the period of study have remained constant. The use of distal aortic perfusion, cerebrospinal fluid drainage, and moderate hypothermia have remained consistent. Intercostal artery reattachment is integral for spinal cord protection, but ultimately has become guided by use of neuromonitoring with motor-evoked potentials (17). Ultimately, this has allowed selective reattachment of intercostal arteries, thus saving operative time while maintaining spinal cord protection. Using this approach, morbidity related to open repair was acceptable, with an incidence of permanent spinal cord injury of 1.3%. What was not determined during the follow-up of this study was the type of medical management administered, as well as patients’ corresponding compliance and the level of benefit achieved (22). This underscores the importance of life-long follow-up in patients with chronic aortic dissection.
In conclusion, the results of the current study should not temper the enthusiasm for advancing endovascular therapies for chronic thoracic aortic dissection. Rather, this study can provide solace in that open repair can be performed with respectable early and late outcomes. Therefore, initial consideration of open repair for complicated CDTAD is appropriate unless patient risks are excessive.
Acknowledgements
The authors thank Chris Akers for his support with medical illustrations.
Disclosure: The authors declare no conflict of interest.
References
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [PubMed]
- Mani K, Clough RE, Lyons OT, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg 2012;43:386-91. [PubMed]
- Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36:522-9. [PubMed]
- Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 2011;142:1074-83. [PubMed]
- Andacheh ID, Donayre C, Othman F, et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg 2012;56:644-50; discussion 650. [PubMed]
- Scali ST, Feezor RJ, Chang CK, et al. Efficacy of thoracic endovascular stent repair for chronic type B aortic dissection with aneurysmal degeneration. J Vasc Surg 2013;58:10-7. [PubMed]
- Nozdrzykowski M, Etz CD, Luehr M, et al. Optimal treatment for patients with chronic Stanford type B aortic dissection: endovascularly, surgically or both? Eur J Cardiothorac Surg 2013;44:e165-74; discussion e174.
- Lee M. Outcomes of endovascular management for complicated chronic type B aortic dissection: effect of the extent of stent graft coverage and anatomic properties of aortic dissection. J Vasc Interv Radiol 2013;24:1451-60. [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [PubMed]
- Brunkwall J, Lammer J, Verhoeven E, et al. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. Eur J Vasc Endovasc Surg 2012;44:31-6. [PubMed]
- Conway AM, Sadek M, Lugo J, et al. Outcomes of open surgical repair for chronic type B aortic dissections. J Vasc Surg 2014;59:1217-23. [PubMed]
- Mutsuga M, Narita Y, Araki Y, et al. Spinal cord protection during a thoracoabdominal aortic repair for a chronic type B aortic dissection using the aortic tailoring strategy. Interact Cardiovasc Thorac Surg 2010;11:15-9. [PubMed]
- Zoli S, Etz CD, Roder F, et al. Long-term survival after open repair of chronic distal aortic dissection. Ann Thorac Surg 2010;89:1458-66. [PubMed]
- Estrera AL, Miller CC 3rd, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg 2005;80:1290-6; discussion 1296. [PubMed]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. [PubMed]
- Estrera AL, Sheinbaum R, Miller CC 3rd, et al. Neuromonitor-guided repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2010;140:S131-5; discussion S142-S146.
- DeBakey ME, Beall AC Jr, Cooley DA, et al. Dissecting aneurysms of the aorta. Surg Clin North Am 1966;46:1045-55. [PubMed]
- Debakey ME, Henly WS, Cooley DA, et al. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 1965;49:130-49. [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [PubMed]
- Dake MD, Thompson M, van Sambeek M, et al. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg 2013;46:175-90. [PubMed]
- Melby SJ, Zierer A, Damiano RJ Jr, et al. Importance of blood pressure control after repair of acute type a aortic dissection: 25-year follow-up in 252 patients. J Clin Hypertens (Greenwich) 2013;15:63-8. [PubMed]