Novel radiation therapy approaches in malignant pleural mesothelioma
Introduction
The pattern of spread of malignant pleural mesothelioma (MPM) poses unique challenges to a radiation oncologist. Since the disease is most often confined to the ipsilateral pleura, local control is the primary concern, particularly after surgical resection. Yet, treating the entire pleura requires a large radiation field, increasing toxicity risks. Initially, when administering radiotherapy (RT) as adjuvant therapy following extrapleural pneumonectomy (EPP) or pleurectomy/decortication (P/D), patients were treated with conventional radiation techniques using anterior and posterior fields. More recently, complex intensity-modulated radiation therapy (IMRT) techniques for the treatment of MPM have been explored, with early outcomes suggesting acceptable safety in appropriately selected patients. Here, we review the lessons learned from conventional radiation techniques and the development of novel radiation techniques for MPM treatment.
Conventional radiation techniques
Traditionally, adjuvant RT has been given through anterior and posterior fields that encompass the entire involved hemithorax. This simple approach has the advantage of avoiding oblique angles that could expose the contralateral lung to low doses of radiation. Sparing of organs in the RT field, e.g., heart, liver, kidneys, or stomach, is achieved by blocking those areas and adding an electron boost to the anterior and posterior chest wall to compensate for the missing dose. This invariably leads to dose uncertainties along the edges of blocked areas as well as under- and overdosing of the pleural space and chest wall. Also, tolerance limits require blocking the spine after 4,140 cGy, often leading to underdosing of the medial pleura and hilum.
Conventional RT after EPP
This technique was first used in patients after EPP, who therefore had no intact lung in the radiation field (1). Blocking was only required for the heart and organs of the upper abdomen (liver and kidney on the right, stomach and kidney on the left), but not within the thorax. A phase II trial exploring high-dose hemithoracic RT to 54 Gy following EPP demonstrated high rates of local control, with only two isolated locoregional failures in 54 patients and a median survival of 17 months (2) (stage I and II tumors, 33.8 months; stage III and IV, 10 months). Multiple subsequent studies incorporated this technique into a multimodality approach combining chemotherapy, EPP, and hemithoracic radiation (3-6). However, with conventional RT there may be radiation underdosing near regions that are blocked. This has the potential to lead to increased risk of local failure in approximately 10-15% of patients (7).
Conventional RT after P/D
A similar hemithoracic RT technique has also been tested after P/D. Since the ipsilateral lung remains in situ after P/D, a block was added for the central part of the lungs. The heart and upper abdominal organs were blocked following the same technique as for patients after EPP. Accordingly the anterior and posterior chest walls located underneath the heart, lung and upper abdominal blocks were boosted with an electron field. Unfortunately, this technique resulted in a disappointing 1-year local control rate of 42% and a median survival of 13.5 months (8). Possible explanations include median radiation dose being only 42.5 Gy, and the dose uncertainties with this technique. In addition, the treatment was quite toxic, with 28% grade 3-4 toxicity and two patients with possible grade 5 cardiac and pulmonary toxicity. A different technique is therefore needed to improve outcomes after P/D.
Novel radiotherapy techniques
IMRT
IMRT is a highly conformal radiation technique that allows more effective sparing of normal tissues, providing an opportunity for safer, less toxic treatments and increased efficacy by enabling higher radiation doses to the tumor target. It comes with a much higher level of dosimetric control and certainty, leading to better target coverage than conventional techniques (9). Areas of under- or overdosing are readily recognizable and can be corrected in the planning phase.
IMRT after EPP
A potential disadvantage of IMRT is the dose of radiation delivered to the contralateral lung, which potentially leads to a higher risk of pneumonitis. Several groups reported significantly increased toxicity and even deaths from radiation pneumonitis in patients treated with IMRT after EPP (10-12). A higher mean lung dose and the volume of lung receiving 5, 10, or 20 Gy have been associated with a greater risk for lung toxicity (11-13). Strict dosimetric guidelines, particularly on the contralateral lung, are therefore paramount. Increasing experience with the technique appears to lead to improved target coverage and decreased toxicity rates (13-16).
IMRT after P/D
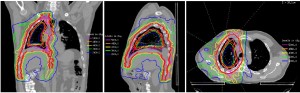
Recently the use of EPP for the surgical management of MPM has declined, due to reports suggesting a lack of survival benefit compared with P/D (17,18). As such, RT techniques will need modification to overcome the challenge of administering it to patients with two intact lungs. A small series investigating palliative IMRT in unresectable MPM reported no severe side effects (19). Recently, we published our initial experience with pleural IMRT in 36 patients with two intact lungs (Figure 1) (20). All patients were planned with a PET/CT scan to aid target delineation. Six to nine coplanar 6 MV beams equispaced over 200-240 degrees around the ipsilateral hemithorax were used. Treatment was delivered in 1.8 Gy fractions up to a total dose of 50.4 Gy. The total delivered dose was driven by the ability to meet normal tissue constraints, in particular a median lung dose <21 Gy. With a median dose of 46.8 Gy, 1- and 2-year survival was 75% and 53%, with a median survival of 26 months in patients who underwent P/D. Seven (20%) patients experienced grade 3 or 4 pneumonitis. These encouraging results have led to an ongoing phase II trial of trimodality therapy using induction chemotherapy with cisplatin and pemetrexed, P/D, and adjuvant pleural IMRT. A failure-pattern analysis of 64 patients treated with this technique revealed that the majority of local failures occurred in sites of gross disease, indicating the need to achieve macroscopically complete surgical resection when feasible (21). Increasing experience over time led to fewer marginal failures and decreased toxicity, suggesting the improvement in target delineation and RT planning.
The higher precision of IMRT requires a detailed knowledge of the anatomy of the thorax and the diaphragm, the incorporation of all diagnostic imaging tools available, information about the pathologic findings at the time of surgery, assessment of the respiratory tumor motion using a 4D scan, and image-guided treatment delivery. IMRT with integration of a boost to areas of gross disease is technically feasible (14,16,22-24), but needs testing in a larger series. The use of 18-fluorodeoxyglucose positron emission tomography (FDG-PET) for RT planning purposes may reduce the likelihood of geographic misses and detect radiographically occult lymph node involvement (25). Small series have suggested that PET may guide the delineation of an integrated boost volume (24) or improve local control (26).
Future radiotherapy techniques
Arc therapy or helical tomotherapy are rotational RT techniques that deliver radiation from even more beam angles than IMRT. They are ideally suited for spherical or circular targets and are therefore of particular interest in MPM. Sterzing et al. reported improved target coverage with tomotherapy compared with IMRT (27). Early clinical results with tomotherapy following EPP suggest high rates of local control, but also showed two grade 5 pneumonitis events (28). We recently compared arc therapy and IMRT plans in patients with two intact lungs and found that arc therapy significantly lowered RT dose to lungs and heart, and shortened the treatment duration (29).
The widespread interest in proton radiation therapy has begun to carry over to MPM. To date, two theoretical planning studies have been published that suggest that proton therapy allows better sparing of normal organs at risk while providing better target coverage in patient after EPP (30,31). However, many uncertainties regarding the feasibility of proton therapy in thoracic RT remain, given the significant impact of respiratory motion and stark tissue density variation in the thorax on accurate proton dosimetry. Clinical validation of proton therapy for MPM remains to be shown.
Conclusions
RT techniques have evolved rapidly in the last decade and continue to be refined. IMRT is currently being explored as adjuvant or definitive treatment in MPM. Severe toxicities have been observed with IMRT, and thus patients should only be treated at high-volume centers with significant experience. Since MPM is rare, as many patients as possible should be enrolled in clinical trials to identify treatment approaches that lead to improved outcomes. Future studies will need to test whether the theoretical advantages of newer RT techniques such as arc or proton therapy can be translated into clinical benefit for MPM patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yajnik S, Rosenzweig KE, Mychalczak B, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2003;56:1319-26.
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95.
- Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006;1:289-95.
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13.
- Pagan V, Ceron L, Paccagnella A, et al. 5-year prospective results of trimodality treatment for malignant pleural mesothelioma. J Cardiovasc Surg (Torino) 2006;47:595-601.
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504.
- Gupta V, Krug LM, Laser B, et al. Patterns of local and nodal failure in malignant pleural mesothelioma after extrapleural pneumonectomy and photon-electron radiotherapy. J Thorac Oncol 2009;4:746-50.
- Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2005;63:1045-52.
- Krayenbuehl J, Oertel S, Davis JB, et al. Combined photon and electron three-dimensional conformal versus intensity-modulated radiotherapy with integrated boost for adjuvant treatment of malignant pleural mesothelioma after pleuropneumonectomy. Int J Radiation Oncology Biol Phys 2007;69:1593-9.
- Allen AM, Czerminska M, Jänne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 2006;65:640-5.
- Kristensen CA, Nøttrup TJ, Berthelsen AK, et al. Pulmonary toxicity following IMRT after extrapleural pneumonectomy for malignant pleural mesothelioma. Radiother Oncol 2009;92:96-9.
- Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys 2008;71:1143-50.
- Rice DC, Smythe WR, Liao Z, et al. Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2007;69:350-7.
- Tonoli S, Vitali P, Scotti V, et al. Adjuvant radiotherapy after extrapleural pneumonectomy for mesothelioma. Prospective analysis of a multi-institutional series. Radiother Oncol 2011;101:311-5.
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3.
- Patel PR, Yoo S, Broadwater G, et al. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:362-8.
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72.
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.
- Münter MW, Thieke C, Nikoghosyan A, et al. Inverse planned stereotactic intensity modulated radiotherapy (IMRT) in the palliative treatment of malignant mesothelioma of the pleura: the Heidelberg experience. Lung Cancer 2005;49 Suppl 1:S83-6.
- Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83.
- Rimner A, Yorke E, McLane A, et al. Failure Patterns after Hemithoracic Intensity-Modulated Radiation Therapy for Malignant Pleural Mesothelioma with Two Intact Lungs. 11th International Conference of the International Mesothelioma Interest Group 2012.
- Ahamad A, Stevens CW, Smythe WR, et al. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2003;55:768-75.
- Cilla S, Digesú C, Silvano G, et al. Intensity-modulated radiation therapy with simultaneous integrated boost in unresected left-sided pleural mesothelioma: a case report. Tumori 2010;96:618-22.
- Fodor A, Fiorino C, Dell’Oca I, et al. PET-guided dose escalation tomotherapy in malignant pleural mesothelioma. Strahlenther Onkol 2011;187:736-43.
- Pehlivan B, Topkan E, Onal C, et al. Comparison of CT and integrated PET-CT based radiation therapy planning in patients with malignant pleural mesothelioma. Radiat Oncol 2009;4:35.
- Feigen M, Lee ST, Lawford C, et al. Establishing locoregional control of malignant pleural mesothelioma using high-dose radiotherapy and [18] F-FDG PET/CT scan correlation. J Med Imaging Radiat Oncol 2011;55:320-32.
- Sterzing F, Sroka-Perez G, Schubert K, et al. Evaluating target coverage and normal tissue sparing in the adjuvant radiotherapy of malignant pleural mesothelioma: helical tomotherapy compared with step-and-shoot IMRT. Radiother Oncol 2008;86:251-7.
- Sylvestre A, Mahé MA, Lisbona A, et al. Mesothelioma at era of helical tomotherapy: results of two institutions in combining chemotherapy, surgery and radiotherapy. Lung Cancer 2011;74:486-91.
- Dumane V, Rimner A, Yorke E, et al. Comparison of Two Different Methods to Deliver Pleural Intensity-Modulated Radiation Therapy in Malignant Pleural Mesothelioma 2012.
- Krayenbuehl J, Hartmann M, Lomax AJ, et al. Proton therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. Int J Radiat Oncol Biol Phys 2010;78:628-34.
- Lorentini S, Amichetti M, Spiazzi L, et al. Adjuvant intensity-modulated proton therapy in malignant pleural mesothelioma. A comparison with intensity-modulated radiotherapy and a spot size variation assessment. Strahlenther Onkol 2012;188:216-25.