Pleurectomy/decortication, chemotherapy, and intensity modulated radiation therapy for malignant pleural mesothelioma: rationale for multimodality therapy incorporating lung-sparing surgery
Introduction
Historically, extrapleural pneumonectomy (EPP) was the preferred surgical approach for the management of malignant pleural mesothelioma (MPM) and has been well studied in the multimodality paradigm. Pleurectomy/decortication (P/D), which spares unaffected lung, is associated with less morbidity and mortality but traditionally was reserved for patients who were not candidates for EPP. However, based on a recent retrospective report as well as changing physician and patient preferences, more lung sparing procedures are now performed. As a result, many investigators are exploring how best to incorporate P/D into a multimodality program.
Multimodality therapy with EPP
EPP is an aggressive surgery associated with significant morbidity and mortality, even when performed at experienced centers (1,2), but it has long been considered potentially curative for MPM. Unfortunately, local recurrence occurs in the vast majority of patients (80%) (2,3) but this risk can be reduced with post-operative radiation therapy. A phase II trial demonstrated a local control rate of 87% after 54 Gy of hemithoracic radiation to the chest cavity after EPP but distant recurrence occurred in 55% of patients (4). Subsequently, with the advent of efficacious chemotherapy regimens for MPM, systemic chemotherapy was added to the treatment paradigm of EPP and hemithoracic radiation. Multiple groups have reported results with trimodality therapy (EPP, radiation, and chemotherapy) with fairly similar outcomes (Table 1): median overall survival ranges from 14 to 28 months and usually 80% of patients successfully undergo EPP (5-16). However, despite these promising results, many patients are unable to tolerate postoperative radiation and/or chemotherapy.
Timing of chemotherapy
Given the success of preoperative chemotherapy for locally advanced non-small cell lung cancer, preoperative chemotherapy was explored to improve the tolerability of therapy for MPM. Preoperative chemotherapy confers several potential advantages, including better tolerance, and the ability to assess response to chemotherapy by imaging. The risk associated with preoperative chemotherapy relates to patients whose MPM progresses during chemotherapy. Surgery may be more difficult or no longer possible in these patients. However, in studies of preoperative chemotherapy in MPM (Table 1), this risk appears small (5-7,9,10,12-14).
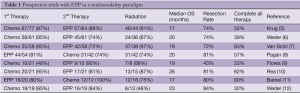
Full table
Surgical approach
Given the challenges of adding radiation and chemotherapy to EPP, the value of EPP as opposed to P/D was examined in a retrospective analysis of 667 patients (17). In a multivariate analysis that controlled for stage, histology, gender, and multimodality therapy, patients who underwent EPP had a poor survival with a hazard ratio of 1.4 compared to P/D. While selection bias may have played a role in the observed difference in this retrospective analysis, this report has had dramatic impact on the practice patterns of many thoracic surgeons as well as on the acceptability of EPP to patients when faced with the possibility of a less extensive surgery that may be equally efficacious. As discussed in this issue by Drs. Rice, Friedberg, Rusch, Sugarbaker, Bölükbas, Waller, and Edwards, both surgical approaches remain important in the management of MPM.
Multimodality therapy with P/D
P/D involves resection of the pleura but leaves the uninvolved lung intact. Although the morbidity and mortality associated with this procedure is less than that with EPP (18-20), P/D has historically been reserved for patients unable to tolerate EPP. The addition of radiation and chemotherapy to EPP has been shown to improve local and distant disease control, as described above. Similarly, as more P/Ds are performed, studies are currently underway to evaluate whether the addition of radiation and chemotherapy to P/D would also improve control of local and distant tumor recurrence. To date, only a single small prospective P/D multimodality series has been reported, with a median overall survival of 30 months (18). Although limited data are available, the complete delivery of all three therapeutic modalities appears more feasible with P/D as compared to EPP. After P/D, 6-16% of patients are unable to complete adjuvant chemotherapy and radiation, compared with 19- 61% after EPP (8,11,15,16,18,21). Early data suggest that by allowing the increased delivery of adjuvant therapy in a greater number of patients, multimodality therapy with P/D is associated with improved survival.
Radiation techniques
As discussed within this issue by Drs. Rimner and Rosenzweig, both conventional and novel radiation techniques have been pursued for the treatment of MPM. There have been significant safety concerns with intensity modulated radiation therapy (IMRT) after EPP (22,23), and as such, exploration of this technique after P/D has progressed cautiously. We recently reported our institutional experience of pleural IMRT in patients with two intact lungs (24). Between 2005 and 2010, 36 patients (with two intact lungs) were treated with pleural IMRT to the hemithorax, using a median dose of 46.8 Gy (range, 41.4-50.4 Gy). Twenty of these patients (56%) had prior lung sparing surgery (pleurectomy/decortication), while 16 (44%) had not had prior resection of mesothelioma. Patient characteristics were typical, with a median age of 67 years (range, 42-82 years) and predominantly epitheloid histology (78%). Staging was distributed as follows: 2 (6%) stage I, 10 (28%) stage II, 12 (33%) stage III, and 12 (33%) stage IV.
Acute severe pulmonary toxicity occurred in 7 patients (20%), all of which was due to pneumonitis. For all but 1 patient, this was reversible with steroids and respiratory support. The single death was possibly due to acute pneumonitis and occurred in an elderly man whose baseline functional and respiratory status were significantly compromised prior to treatment. The only other observed severe toxicity was grade 3 fatigue in 2 patients (6%). Survival was encouraging: with a median follow up of 18 months, median overall survival was 18 months (95% CI, 15-33 months). Among those who underwent P/D in addition to pleural IMRT, median overall survival was 26 months, with 75% and 53% survival at 1 and 2 years, respectively. Overall, treating MPM patients with intact lungs, using pleural IMRT, is feasible and safe, with a reasonable rate of severe toxicity compared with other therapeutic options. It is also associated with encouraging survival rates in this retrospective cohort.
Novel multimodality paradigm
Based on our encouraging institutional experience with pleural IMRT and the increasing population of MPM patients who undergo P/D, we have an ongoing prospective multicenter phase II trial evaluating the safety and efficacy of pre-operative chemotherapy, P/D (if feasible), and pleural IMRT (Figure 1). Chemotherapy-naïve patients with MPM receive up to 4 cycles of pemetrexed-platinum chemotherapy, followed by P/D (if feasible) and pleural IMRT. Eligibility criteria include no metastatic disease, no prior radiation therapy except for localized prostate or pelvic radiation, and an FEV1 ≥30% of predicted postoperative. Patients who require EPP are ineligible. The primary endpoint of this study is grade 3 or greater pneumonitis. Grade 3 pneumonitis, as defined by the National Cancer Institute Common Terminology Criteria for Toxicity and Adverse Event Reporting version 4.0, is symptomatic respiratory dysfunction, interfering with activities of daily living and requiring oxygen support. The trial employs a two-stage design. In the first stage with 9 patients, if 3 or more patients experience grade 3 or greater pneumonitis, the trial will stop. If 2 or fewer of the 9 patients experience grade 3 or greater pneumonitis, enrollment will be extended to 28 evaluable patients. If 5 or fewer of 28 evaluable patients experience grade 3 or greater pneumonitis, the regimen will be considered safe and feasible. Secondary endpoints include response rate to chemotherapy, progression free survival, overall survival, and patterns of progression. Accrual should be completed within the year.
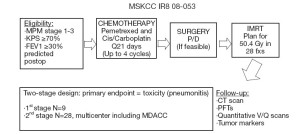
Conclusions
MPM patients clearly benefit from a multimodality approach, but the optimal combination of surgery, chemotherapy, and radiation remains unknown. As more patients undergo P/D for early-stage disease, the wellstudied multimodality paradigm for EPP is being modified to incorporate P/D instead. Advances in radiation therapy have allowed the exploration of novel techniques for patients with 2 intact lungs. Our preliminary retrospective analysis demonstrated the safety of pleural IMRT after P/D, and we are currently evaluating this novel paradigm of preoperative chemotherapy, P/D, and pleural IMRT in a prospective phase II trial.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chang MY, Sugarbaker DJ. Extrapleural pneumonectomy for diffuse malignant pleural mesothelioma: techniques and complications. Thorac Surg Clin 2004;14:523-30.
- Rusch VW, Piantadosi S, Holmes EC. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J Thorac Cardiovasc Surg 1991;102:1-9.
- Pass HI, Kranda K, Temeck BK, et al. Surgically debulked malignant pleural mesothelioma: results and prognostic factors. Ann Surg Oncol 1997;4:215-22.
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95.
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13.
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202.
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9.
- Pagan V, Ceron L, Paccagnella A, et al. 5-year prospective results of trimodality treatment for malignant pleural mesothelioma. J Cardiovasc Surg (Torino) 2006;47:595-601.
- Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006;1:289-95.
- Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): Feasibility and results. Lung Cancer 2007;57:89-95.
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504.
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22:3451-7.
- Buduhan G, Menon S, Aye R, et al. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 2009;88:870-5; discussion 876.
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8.
- Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6.
- Okubo K, Sonobe M, Fujinaga T, et al. Survival and relapse pattern after trimodality therapy for malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2009;57:585-90.
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.
- Bölükbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/ Pemetrexed and radiotherapy. Lung Cancer 2011;71:75-81.
- Nakas A, Martin Ucar AE, Edwards JG, et al. The role of video assisted thoracoscopic pleurectomy/decortication in the therapeutic management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;33:83-8.
- Rusch VW. Pleurectomy/decortication in the setting of multimodality treatment for diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 1997;9:367-72.
- Maggi G, Casadio C, Cianci R, et al. Trimodality management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2001;19:346-50.
- Allen AM, Czerminska M, Jänne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 2006;65:640-5.
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3.
- Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83.





