Standardizing surgical treatment in malignant pleural mesothelioma
Of all thoracic malignances, the management of mesothelioma is perhaps the most controversial, and this is especially true with respect to surgical treatment. Mesothelioma is a relatively rare disease with only 2,500 new cases being diagnosed each year in the United States, making it difficult to perform large scale prospective, let alone randomized studies that can provide level I evidence. The disease is extremely heterogeneous, with histological subtype having a major impact on overall survival. Even within individual tumors, there may be regional variation in the percentages of epithelioid to non-epithelioid components, making pretreatment biopsies often inaccurate. Beyond tumor subtype, the natural history of the disease often differs from patient to patient. Some patients present with relatively low volume indolent disease, whereas others have bulky tumors that behave in a much more aggressive fashion.
To date, clinical staging for mesothelioma has proven to be extremely inaccurate. Part of the reason for this has been attempts to extrapolate surgical staging parameters to the clinical setting. Unfortunately, our ability to measure these parameters (nodal involvement, chest wall invasion, pericardial invasion, etc.) has been restricted by the inaccuracy of current imaging technology.
For all these reasons, much of our understanding of this disease comes from either single-institution retrospective studies or from prospective single-or multi-institutional phase I or II trials, most of which only involved small numbers of patients. Given the need to make comparisons between studies to develop treatment strategies, it is ideal then to be able to compare like with like, apples with apples. General consensus among authors regarding which parameters and endpoints to consistently report will go a long way towards being able to allow meaningful interstudy comparisons to be made. This is particularly relevant with respect to surgical nomenclature.
It is widely accepted by most surgeons who operate on mesothelioma that the goal of surgery with a curative intent should be macroscopic complete resection (MCR) of the tumor (1). This paradigm is consistent with the surgical treatment of most other solid malignancies: surgery provides macroscopic removal of the primary tumor while adjunctive therapies such as chemotherapy and radiation help address local microscopic residual tumor or distant micrometastatic disease.
Two surgical techniques exist for achieving MPM MCR - extrapleural pneumonectomy (EPP), or pleurectomy/ decortication (P/D) and extended pleurectomy/decortication (EPD). The former involves resection of the ipsilateral lung, visceral and parietal pleura, ipsilateral hemidiaphragm and pericardium with reconstruction of the latter two structures to prevent cardiac and visceral herniation. The components of the operation and details of the technique have been extensively described in the published literature (2,3). Accordingly, when surgeons report outcomes of EPP we understand exactly the procedure that those patients underwent and this allows comparison of outcomes to be made taking into account, of course, other important patient/tumor variables.
Unfortunately, the same cannot be said for P/D, which is an operation that has yet to be standardized (4). In mesothelioma surgery ‘pleurectomy/decortication’ may mean different things to different surgeons. There are some who assume it to mean an operation that completely removes all visible or palpable tumors from the thoracic cavity (i.e., MCR) while preserving the lung parenchyma, with the goal of survival prolongation. Often, in order to remove all gross tumors, this may necessitate resection of the pericardium or diaphragm, similar to EPP. On the other hand, some surgeons label a procedure P/D when it only involves partial stripping of the parietal pleura and decortication of sufficient visceral pleura to facilitate expansion of the underlying trapped lung. In this setting, parietal and visceral pleura have been removed, but the goal is not macroscopic complete resection but rather the palliation of symptoms. Occasionally, operative reports of P/D will describe an operation where very little more than a generous parietal pleural biopsy has been performed. There is, therefore, a degree of ambiguity about the extent of the procedure described in many published reports of P/D, unless the authors have taken the time to specifically outline their definition of the procedure and the therapeutic intent (5).
In 2008, the International Mesothelioma Interest Group (IMIG) collaborated with the International Association for the Study of Lung Cancer (IASLC) to address deficiencies in the staging system for mesothelioma. The Mesothelioma Domain was established as part of the IASLC Staging Committee, with the mission of revising the current (and inadequate) TNM staging system for mesothelioma, similar to the process that had been performed several years earlier for lung cancer. Because the existing staging system was based solely on data obtained retrospectively from surgically treated patients, the Mesothelioma Staging Project was designed to prospectively collect data on patients with mesothelioma regardless of treatment modality so that a clinical staging system could be derived that would be relevant in the pre-therapy setting and be applicable to both surgical and non-surgical patients. As surgical treatment would be one of the variables collected and analyzed, it was evident that strict definitions of types of surgery applied would be important for uniform collection and correct interpretation of data. Accordingly, a multinational survey of surgeons who actively practiced cytoreductive surgery for mesothelioma was performed to better understand current concepts regarding the extent of surgery that is performed for mesothelioma and the nomenclature of those operations (6).
A number of important findings were identified from this survey. Firstly, most (88%) respondents agreed that the goal of cytoreductive surgery in mesothelioma should be MCR. Secondly, the majority of respondents (95%) felt that there was a need to refine surgical nomenclature to reflect procedural differences between P/D performed for palliation and P/D performed for MCR. Thirdly, P/D was considered resection of the parietal and visceral pleura (with or without the diaphragm or pericardium) with the aim of achieving MCR by a majority (72%) of respondents. Finally, in cases of P/D where diaphragm and/or pericardium required resection, “radical P/D” was the term favored to describe this approach by 64%, whereas “P/D” (40%) or “total pleurectomy” (39%) were the preferred terms when these structures were not removed. On the basis of this survey, the IASLC Staging Committee and the Mesothelioma Domain recommended that the following terminology to be used in the Mesothelioma Staging Project:
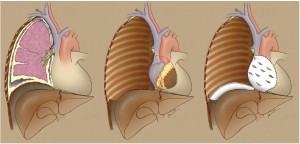
(I) Extrapleural pneumonectomy (EPP): en bloc resection of the parietal and visceral pleura with the ipsilateral lung, pericardium, and diaphragm. In cases where the pericardium and/or diaphragm are not involved by tumor, these structures may be left intact (Figure 1).
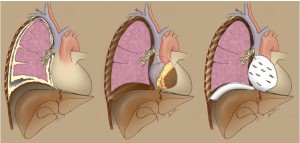
(II) Extended pleurectomy/decortication (EPD): parietal and visceral pleurectomy to remove all gross tumor with resection of the diaphragm and/or pericardium (the IASLC Mesothelioma Domain suggested use of the term “extended” rather than “radical” in this instance as the latter implies a completeness of resection with added therapeutic benefit and there was insufficient evidence that resection of the pericardium and diaphragm provides either) (Figure 2).
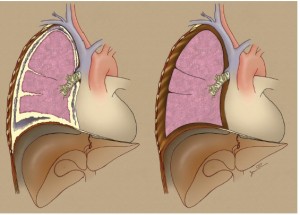
(III) Pleurectomy/decortication (P/D): parietal and visceral pleurectomy to remove all gross tumor without diaphragm or pericardial resection (Figure 3).
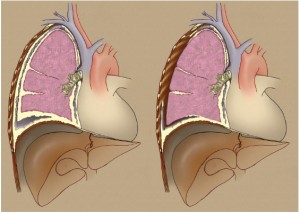
(IV) Partial pleurectomy: partial removal of parietal and/ or visceral pleura for diagnostic or palliative purposes but leaving gross tumor behind (Figure 4).
It should be noted that the purpose of the IASLC in making these recommendations was to standardize surgical nomenclature for reporting cases included in the prospective Mesothelioma Staging Project, and not to imply that this terminology necessarily be adopted among mesothelioma surgeons in general. Nevertheless, the definitions were based on a consensus of expert opinions and may represent a useful framework to better classify surgical procedures for MPM that could reduce some of the ambiguity in the future literature.
Beyond procedural nomenclature, there are two other surgical related issues that may merit some form of standardization. The first pertains to the definition of MCR and the quantification of residual tumor deposits. As mentioned previously, MCR is now accepted as the goal of cytoreductive surgery for pleural mesothelioma, but what exactly constitutes ‘complete’ resection (7)? On the face of it, resection of all macroscopic disease should entail an operation that leaves no evidence of visible or palpable tumor behind (R0/R1), and a great many surgeons ascribe to this view (8,9). However, it is also widely recognized that it is sometimes impossible not to leave behind some small tumor deposits, even after a very aggressive P/D or EPP procedure. If cytoreduction has been performed and small areas of tumor nodularity remain on the endothoracic fascia, should that be considered MCR? The literature varies regarding what constitutes an ‘adequate’ cytoreduction. For instance, in the phase II study by Richards and colleagues, ‘MCR’ included resections where residual amounts of tumor up to 1 cm3 may have remained (10). Likewise, in a phase III study of photodynamic therapy following EPP or P/D, Pass and colleagues attempted maximal cytoreduction employing a cut-off for tumor residuals of ≤5 mm at any intrathoracic site (there could be multiple) (11). Likewise, Rusch and colleagues used ‘microscopic or minimal (<5 mm in thickness) gross residual tumor’ as the arbiter for maximal cytoreduction (12). Others, however, have chosen to interpret MCR literally as an R0/R1 resection where no gross tumor residuals remain (9,13). So clearly, there are differences among surgeons regarding the definition of MCR.
There is also imprecision involved in the surgeon’s ability to accurately estimate the amount of residual disease that remains following a cytoreductive surgery and this information is rarely, if ever, reported in published series. What is clear from research in peritoneal malignancies is that the volume of residual disease correlates with overall and progression-free survival (14). In an effort to quantify and classify residual tumor deposits in peritoneal malignancies, several scoring systems have been developed based on the estimated volume of tumor remaining (15). The most widely accepted is the Completeness of Cytoreduction Score (or CC score, Table 1). The development of a similar scoring system for pleural mesothelioma might aid in stratifying patients in terms of their risk for subsequent recurrence and survival, and would allow more meaningful comparisons to be made between published reports.
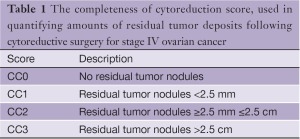
Full table
The second issue is that of standardizing lymph node sampling at the time of cytoreductive surgery. For patients with lung cancer, there is a standardized lymph node map and systematic lymph node sampling or dissection is a well-established practice among general thoracic surgeons. The same is not true in the case of mesothelioma, though the Mesothelioma Domain of the IASLC Staging Committee is actively devising a lymph node map for mesothelioma [personal communication V. Rusch]. As lymph node involvement is a major prognostic indicator in mesothelioma, it is vital that nodal assessment be accurate and thorough. Similar to lung cancer, nodal metastases from mesothelioma may involve the classic N1 and N2 level nodes, but unlike lung malignancies pleural mesothelioma also frequently involves internal mammary, diaphragmatic, anterior mediastinal and even intercostal nodes (16-18). None of these nodal stations are included in the standard systematic node dissection that is performed for lung cancer. Furthermore, these nodes may not be obviously enlarged on preoperative studies or even by visual inspection intraoperatively, though they may still harbor metastases. In addition, there is evidence that the number of positive nodes is predictive of survival (16,18). It stands to reason, therefore, that nodal sampling at the time of cytoreductive surgery should be thorough and extensive so that assessment of prognosis can be as accurate as possible. A standardized approach to sampling intra- and extrapleural nodes during cytoreductive surgery would greatly improve the accuracy and validity of pathologic staging in mesothelioma.
In conclusion, standardization of operative approaches and surgical nomenclature, agreement on what constitutes MCR and development of a method to categorize tumor residuals, and formalization of lymph node sampling will improve our ability to make meaningful comparisons between studies. In addition, this will also provide uniform descriptors to be used in future research, and improve pathologic staging and our efforts to provide accurate prognosis to patients.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72.
- Wolf AS, Daniel J, Sugarbaker DJ. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:132-48.
- Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619-24.
- Pass H. Surgery and mesothelioma: if not randomization, at least standardization and registration! Lung Cancer 2011;71:1-2.
- Friedberg JS, Mick R, Stevenson J, et al. A phase I study of Foscan-mediated photodynamic therapy and surgery in patients with mesothelioma. Ann Thorac Surg 2003;75:952-9.
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol 2011;6:1304-12.
- Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lungsparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886-91.
- Lang-Lazdunski L, Bille A, Belcher E, et al. Pleurectomy/ decortication, hyperthermic pleural lavage with povidoneiodine followed by adjuvant chemotherapy in patients with malignant pleural mesothelioma. J Thorac Oncol 2011;6:1746-52.
- Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93:1658-65; discussion 1665-7.
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7.
- Pass HI, Temeck BK, Kranda K, et al. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol 1997;4:628-33.
- Rusch V, Saltz L, Venkatraman E, et al. A phase II trial of pleurectomy/decortication followed by intrapleural and systemic chemotherapy for malignant pleural mesothelioma. J Clin Oncol 1994;12:1156-63.
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/ decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43.
- Luyckx M, Leblanc E, Filleron T, et al. Maximal Cytoreduction in Patients With FIGO Stage IIIC to Stage IV Ovarian, Fallopian, and Peritoneal Cancer in Day-to- Day Practice: A Retrospective French Multicentric Study. Int J Gynecol Cancer 2012;22:1337-43.
- Braicu EI, Sehouli J, Richter R, et al. Primary versus secondary cytoreduction for epithelial ovarian cancer: a paired analysis of tumour pattern and surgical outcome. Eur J Cancer 2012;48:687-94.
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7.
- de Perrot M, Uy K, Anraku M, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2007;133:111-6.
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2008;136:605-10.