Thoracoabdominal aortic aneurysm
Historical perspective
One of the first successful repairs of a thoracoabdominal aortic aneurysm (TAAA) in the United States was reported in 1955 by Etheredge (1). Utilizing a 5-mm aortic shunt, an in situ aortic homograft repair of an extent IV aneurysm was performed via a thoracoabdominal incision (Video 1). This included anastomoses of the celiac and superior mesenteric arteries. That same year, Charles Rob, an English surgeon, also reported on his experience of 33 abdominal aortic aneurysms, six of which required lower thoracic aortic clamping approached via a thoracoabdominal incision. Rob’s report predated Etheredge’s manuscript, and he is credited with the first description of a TAAA repair (2). Cooley and DeBakey’s initial report of a descending thoracic aortic aneurysm (likely an extent I) approached via a thoracoabdominal incision in 1953 predated Etheredge’s manuscript as well (3), but this repair did not involve direct manipulation of the abdominal visceral arteries and thus Etheredge and Robb remain the first surgeons to describe such a technique. Shortly thereafter, DeBakey reported on four cases similar to Etheredge’s patient utilizing aortic homograft as the conduit, concluding that aortic shunting was imperative to success (4). His subsequent report in 1965 included 42 patients in whom knitted Dacron grafts were utilized as the initial shunt and then converted to the formal conduit by stepwise side branch anastomoses of the visceral arteries (5). Most of these initial reports involved aneurysmectomy which prolonged operative times and led to significant morbidity, and Crawford is credited with pioneering the technique of an intra-aortic anastomosis after longitudinal division of the sac. In addition, he also described the technique of a single pedicled visceral segment anastomosis including the celiac, superior mesenteric, and right renal arteries, followed by a left renal anastomosis with sequential reperfusion after the completion of each (6). His results were superb with only one death in 23 consecutive cases, a mortality rate that is rarely achieved in modern practice. Along with the utilization of cardiopulmonary bypass, hypothermic circulatory arrest, and cerebrospinal fluid drainage, Crawford’s approach most resembles contemporary techniques performed at major centers today.
Definition, etiology, and risk factors
Thoracoabdominal aortic aneurysms result from continuous dilation of the descending thoracic aorta extending into the abdominal aorta. Multiple configurations occur anywhere along the continuum from the origin of the left subclavian artery to the aortoiliac bifurcation. The medial layer of the aortic wall, comprised mainly of structural proteins such as collagen and elastin, contributes to aortic capacitance and elasticity. Degradation of these structural proteins or a defect in their composition leads to medial degeneration and weakening of the aortic wall. Subsequent dilatation results from hemodynamic forces on the arterial wall as well as intrinsic changes in the composition of the arterial wall itself. As defined by the law of Laplace, wall tension is proportional to the pressure multiplied by the radius of the arterial conduit; thus, as the diameter of the aorta increases, wall tension increases, creating a vicious cycle. Hypertension clearly exacerbates this process.
Several genetic disorders are known etiologies of aortic aneurysms. Patients with Marfan syndrome, an autosomal dominant condition resulting in abnormal fibrillin, commonly develop aortic aneurysms. Ehlers-Danlos syndrome, a collagen disorder, also causes similar clinical findings in some patients. Other disorders associated with aortic aneurysms include Turner’s syndrome, polycystic kidney disease, Loeys-Dietz syndrome, syphilis, arteritis, and traumatic injury (7).
It has been postulated that atherosclerosis plays a role in aneurysmal formation as well, and it is clear that if this relationship is not causal, the two conditions occur simultaneously in the majority of patients. The vast majority of TAAAs are the result of atherosclerotic disease, and these are deemed ‘degenerative’. Consequently, risk factors for TAAAs are similar to those for atherosclerosis itself and include smoking, hypertension, obesity, hyperlipidemia, chronic obstructive pulmonary disease (COPD), and family history. It is important to note, however, that in contrast to abdominal aortic aneurysms (AAA) in which the presence of concomitant coronary artery disease (CAD) is greater than 70%, patients with TAAAs have a much lower incidence of CAD, often cited as less than 30%. This seems to indicate that the respective etiologies of aortic dilatation differ somewhat between the thoracic and the abdominal aorta (8,9).
Aortic dissection is another risk factor for development of TAAAs with up to 40% of patients with chronic dissection eventually requiring repair (10). Hypertension, specifically diastolic blood pressure greater than 100 mmHg, seems to be the most consistent risk factor associated with dissection progressing to aneurysm formation (11,12).
Natural history and incidence
The natural history of aortic aneurysms is dissection or rupture. Population studies have revealed the incidence of thoracic aortic aneurysms to be in the range of 10 new aneurysms per 100,000 person-years (13). Up to 80% of these will eventually rupture, owing to a 10-20% five-year survival of patients who remain untreated. Females generally develop TAAAs later in life than men but are at a higher risk of rupture, and in both sexes advanced age is also associated with a higher risk. With respect to comorbidity, COPD had been shown to significantly increase the odds of rupture by 3.6 times (14). The transverse diameter of the aneurysmal aorta is directly related to the risk of rupture. It has been shown that for every 1 cm of growth over 5 cm in the descending thoracic aorta, the risk of rupture nearly doubles (14), resulting in an annual rupture risk for aneurysms greater than 6 cm of 7% (15). Among patients with TAAAs greater than 7 cm, 43% will eventually progress to dissection or rupture (16).
Classification
In 1986, Crawford described the first TAAA classification scheme based on the anatomic extent of the aneurysm (17). Type I involves most of the descending thoracic aorta from the origin of the left subclavian to the suprarenal abdominal aorta. Type II is the most extensive, extending from the subclavian to the aortoiliac bifurcation. Type III involves the distal thoracic aorta to the aortoiliac bifurcation. Type IV TAAAs are limited to the abdominal aorta below the diaphragm. Safi’s group modified this scheme by adding Type V, which extends from the distal thoracic aorta including the celiac and superior mesenteric origins but not the renal arteries (18). See Figure 1.
Indications for repair
While it may seem obvious, it is important to note that all symptomatic aortic aneurysms regardless of size or anatomic extent should be addressed surgically. These symptoms most commonly present as pain or pressure. In the case of the descending thoracic aorta, aneurysmal pain is often described as intrascapular or chest pain radiating to the back, with a ‘tearing’ or ‘stabbing’ quality. Unfortunately, very few patients present with symptoms prior to an acute aortic event, with up to 95% of the events occurring in the absence of any heralding symptoms (19).
The size criteria for repair of asymptomatic TAAAs have been extensively debated in the literature, with groups advocating elective intervention at anywhere from 5 to 10 cm. Elefteriades et al. have published extensively on the natural history and rupture risk of the thoracic aorta stratified by diameter and provide the following guidelines for repair of the descending thoracic aorta (20):
- Rupture
- Acute dissection resulting in malperfusion or other life altering complications
- Symptomatic states
- Pain consistent with rupture and unexplained by other causes
- Compression of adjacent organs
- Documented enlargement ≥1 cm/year or substantial growth approaching absolute size criteria
- Absolute size >6.5 cm or >6.0 cm in patients with connective tissue disorders
Absolute size criteria must be adjusted in patients of extreme size, and nomograms are available to assist the surgeon in decision making for these patients. It is also important to realize that the size criteria above are based on the premise that the appropriate operative time is realized when the annual risk of rupture exceeds the mortality of the proposed procedure. Surgeons at individual institutions must weigh this fact in conjunction with their own mortality rates to best guide the appropriate therapy.
Repair strategies for TAAA
Preoperative workup
Open, endovascular, and hybrid repairs of TAAAs have all been extensively described. Regardless of the operative approach, an extensive preoperative workup is mandatory. In addition to stratifying cardiovascular risk factors, careful attention must be paid to pulmonary and renal function. Most open repairs require single lung ventilation, so preoperative spirometry is recommended, especially in patients with COPD. Postoperative renal failure has been shown to be the most significant risk factor for early mortality in elective TAAA repair, increasing this risk threefold (21), and thus careful attention must be paid to pre-existing renal dysfunction. Some authors advocate calculation of the patient’s preoperative glomerular filtration rate as a more sensitive predictor of postoperative renal failure (22). Endovascular repairs mandate an even higher index of suspicion with regard to preoperative renal function given the use of nephrotoxic contrast agents during these procedures.
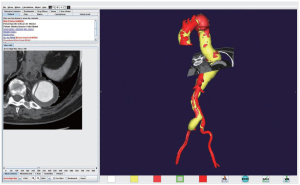
Computed tomographic angiography (CTA) of the aorta with three dimensional reconstruction has become the gold standard for preoperative imaging. This modality is extremely useful in preoperative planning to aid in selection of appropriate repair strategies, especially when considering endovascular or hybrid repairs. The ability to manipulate the 3D image while simultaneously viewing the axial slice is an invaluable tool to assess aneurysmal anatomy. Figure 2 depicts a 3D reconstructed CTA image with perpendicular axial slice designation of an extent II TAAA with chronic dissection.
Anesthesia and intraoperative monitoring
After induction of anesthesia and insertion of a double lumen endotracheal tube, central access is obtained and a pulmonary artery catheter is inserted for hemodynamic monitoring. A Foley catheter is placed, and arterial lines are inserted in both the upper and lower extremities to monitor both proximal and distal perfusion during aortic clamping in cases where left heart bypass (LHB) is used. Lumbar cerebrospinal fluid (CSF) drains are used routinely for extent I and II repairs, maintaining the intrathecal pressure at 10 mmHg or less. In the majority of cases, and especially in cases of extent II repair or those requiring hypothermic circulatory arrest, cranial and peripheral electrodes are placed for monitoring of somatosensory or motor evoked potentials to assess intraoperative spinal cord protection and perfusion. In the case of motor evoked potentials, special anesthetic considerations are required because the use of neuromuscular blocking agents can confound monitoring.
Circulatory support
With regard to extracorporeal bypass or circulatory support for TAAA repair, a variety of strategies are employed. Most groups advocate LHB at a minimum, and it is generally accepted that decompression of the proximal circulation in conjunction with distal perfusion of the abdominal viscera, spinal cord, and lower extremities ameliorates complications associated with ischemia such as generalized metabolic acidemia, acute tubular necrosis or overt renal failure, and paraplegia. This is undertaken by cannulation of the inferior pulmonary vein and any site distal to the aortic clamp site, i.e. from the descending thoracic aorta to the common femoral artery. Our preferred method of LHB includes left atrial drainage via the left inferior pulmonary vein and arterial inflow by cannulation of the iliac system after exposure of the bifurcation. This provides adequate retrograde flow to the visceral segment and the spinal cord via the internal iliac system and antegrade flow to the bilateral lower extremities.
At our institution we more commonly employ partial cardiopulmonary bypass, electing to cannulate the left femoral vein with an extended venous cannula that is advanced to the level of the right atrium and arterial inflow as described above. This strategy avoids issues with right heart strain and is especially advantageous in patients with inadequate pulmonary reserve who may not tolerate single lung ventilation. We believe the advantages of this approach outweigh the additional inflammatory insult resulting from membrane oxygenation. In cases where a proximal clamp site is not feasible, and thus circulatory arrest is mandatory for construction of the proximal anastomosis, we utilize the same cannulation strategy but proceed to hypothermic circulatory arrest with subsequent total body retrograde perfusion. This enables a uniform strategy for all TAAA repairs, streamlining the process for the surgeon and the entire operating room staff.
Organ protection in open TAAA repair
In addition to circulatory support, multiple adjunctive measures to combat ischemic injury of the abdominal viscera and the spinal cord are advocated. These include permissive hypothermia, cold selective renal perfusion, aggressive reattachment of segmental arteries, and sequential aortic clamping in the repair of any TAAA. Additionally, cerebrospinal fluid drainage and selective perfusion of the celiac and superior mesenteric axes are employed in extent I and II TAAA repairs (23).
The protective effects of hypothermia are attributed to decreased metabolic activity and oxygen demand and are generally well accepted. Permissive or active systemic hypothermia to 32 , depending on the method of circulatory support, is universal. Additionally, Coselli and others advocate cold crystalloid (4 ℃) renal perfusion citing a reduction in postoperative acute renal failure (24). Cold crystalloid perfusion of either the intrathecal space or segmental arteries to achieve selective spinal cord hypothermia has been described as well but is not performed at most centers.
Cerebrospinal fluid drainage is another well established strategy to reduce perioperative spinal cord ischemia and resultant paraplegia. An upper lumbar drain is placed preoperatively and CSF is intermittently drained to maintain the intrathecal pressure at or below 10 mmHg. This adjunct is continued into the postoperative period until the patient has manifested intact lower extremity neurologic function. The incidence of postoperative paraplegia is significantly reduced by this method, as multiple studies including one meta-analysis have shown (25). Selective visceral perfusion is also advocated by some authors. This is accomplished by utilizing balloon-tipped catheters and a separate arterial inflow circuit from the bypass pump to perfuse the celiac and superior mesenteric vascular beds with oxygenated blood, particularly when performing the reattachment of proximal thoracic aortic segmental arteries.
In addition to the above adjunctive measures, certain operative techniques can also be utilized to reduce ischemic time and enhance spinal cord perfusion. These include sequential clamping of the aorta to enable immediate reperfusion of segmental arteries and the visceral segments as soon as these anastomoses are completed. It is generally accepted that perfusion to these vascular beds should be reinitiated prior to completing the distal anastomosis in large open repairs, as the lower extremities are clearly more tolerant of prolonged ischemia when compared to the abdominal viscera and the spinal cord. Spinal cord perfusion is augmented by aggressive reattachment of segmental arteries between T8 and L1.
Surgical approach and exposure of the thoracoabdominal aorta
For any extent TAAA repair the patient is placed in the right lateral decubitus position with the table broken at the waist. The shoulders are rotated posteriorly 10-20° and the pelvis is rotated 50-60° posteriorly to allow access to both groins if needed. Access to the descending thoracic aorta and distal arch is gained by a posterolateral thoracotomy through the sixth intercostal space. The fifth space may be appropriate when improved exposure of the distal arch and left subclavian is required; conversely, the eighth or ninth space may be utilized to approach extent III aneurysms with little to no thoracic component. The medial aspect of the thoracotomy incision is carried across the costal margin in a gentle curve and proceeds inferiorly to the left of midline to just below the level of the umbilicus for the retroperitoneal approach. Care should be taken upon entering the abdomen to clearly identify the external and internal oblique layers, as their identification aids in closure later in the case. Following division of the internal intercostal and transversus abdominis layers, meticulous blunt dissection is used to avoid entering the peritoneal sac. After the costal margin is divided, the diaphragm is incised circumferentially taking care to avoid injury to the phrenic nerve but leaving enough diaphragmatic cuff on the chest wall to facilitate closure. Fixed retractors are used to provide exposure, and the peritoneal sac and its contents along with the left kidney can be reflected rightward and anteriorly by bluntly developing the plane between the sac itself and the lateral abdominal wall. This approach should provide excellent exposure to the entire length of the thoracoabdominal aorta.
Repair of the descending thoracic aortic segment
After heparinization and the initiation of either left heart or cardiopulmonary bypass as described above, the distal arch is mobilized by dividing the ligamentum arteriosum, taking care to avoid injury to the vagus and recurrent laryngeal nerves. The proximal aortic clamp is placed between the left common carotid and subclavian artery origins, or on the proximal descending thoracic aorta, depending on the extent of the proximal involvement of the aneurysm. A distal clamp is placed in the region of T4-T6, the aorta is opened, and patent segmental arteries in this region are oversewn. The aorta is then divided at the level of the proximal anastomosis and freed from surrounding tissues including the esophagus. A woven Dacron tubular graft is selected after sizing the aorta, and the anastomosis is performed using a running 3-0 polypropylene suture. At our institution the proximal clamp is usually left in place for the remainder of the operation unless it was placed proximal to the takeoff of the left subclavian, to avoid any possibility of retrograde embolism to the cerebral circulation.
Special considerations are required in patients necessitating circulatory arrest because of aneurysmal disease of the arch, rupture, aortic size or calcification. After cooling to the appropriate level, the proximal anastomosis is performed in the same fashion under circulatory arrest with subsequent de-airing of the arch and re-establishment of antegrade perfusion prior to proceeding with the distal anastomosis. It is also important to note that in patients with concurrent ascending or aortic arch aneurysmal disease who have not undergone a previous elephant trunk repair, the proximal portion of the Dacron graft can be involuted into itself to facilitate the distal arch anastomosis at the time of the staged second operation.
Repair of the abdominal aortic segment
After completion of the proximal anastomosis, the abdominal segment is clamped 2-3 cm below the proposed distal anastomotic site and the aorta is opened longitudinally posterior to the origin of the left renal artery. The origins of the visceral and renal arteries are identified and endarterectomized if necessary. Selective perfusion with oxygenated blood or cold crystalloid, respectively, is performed at this time. A patch of patent segmental arteries is identified and sewn to a corresponding opening in the graft with a continuous 4-0 polypropylene suture. A visceral patch including the celiac, superior mesenteric, and right renal arteries is fashioned and sewn to the graft in the same fashion, except in patients with connective tissue disorders who require separate branch graft anastomoses to avoid the possibility of developing visceral patch aneurysmal dilatation. The left renal artery requires a separate branch graft anastomosis as well. The clamp can be progressively moved distally as anastomoses are completed to sequentially reperfuse organ beds.
Variations exist depending on the extent of the TAAA. Extent I aneurysms can usually be repaired with a beveled anastomosis to the abdominal aorta just above the visceral segment. Extent V aneurysms can be repaired in the same fashion with a beveled anastomosis including the bilateral renal arteries. Figure 3 includes representations of open repair techniques of extent I-V TAAAs.
Operative technique of endovascular TAAA repair
Total endovascular repair of TAAAs is still in the experimental phase worldwide, and to date there are no commercially available fenestrated or branched endografts available in the United States for TAAAs. Initially, enthusiasm for total endovascular repair of TAAAs was lacking due to the fear of spinal cord ischemia resulting from the exclusion of intercostal and lumbar arteries, but these fears have waned with the progression of successful endovascular treatment of juxtarenal AAAs. As with all endovascular therapies, European and Australian studies predated those in the United States. The first American series of Cook’s (Cook Medical Inc., Bloomington, IN) branched endografts was published in 2007 and reported a 93% technical success rate and a 6% mortality rate (26). These grafts are custom made specifically for each patient’s aortic anatomy, however, requiring more than a month for fabrication. Phase I trials are ongoing for “off-the-shelf” grafts with prefabricated fenestrations or branches. Other currently available options include “back table” alterations of commercially available grafts, but this is a highly surgeon- or center-specific skill, making it exceedingly difficult for universal application. “Snorkel” techniques have also been advocated for aneurysms with a limited thoracic component, but currently long-term data is lacking.
Operative technique of hybrid TAAA repair
The limited role of total endovascular repair for TAAAs has led to the development of “hybrid” or “debranching” repairs. These repairs are often staged. Brachiocephalic and visceral branches of the aorta are debranched to the extent necessary depending on the patient’s anatomy. Carotid to subclavian bypass or retrograde visceral debranching from the common iliac arteries is employed prior to endovascular exclusion of the TAAA. Theoretical advantages include obviating the need for thoracotomy in patients with significant pulmonary comorbidity and avoiding prolonged ischemic times associated with open repair. In a retrospective comparison to open repair, early reports claim reduced perioperative complication rates with similar long-term, but no prospective comparisons are available to date (27).
Outcomes in open TAAA repair
The most favorable results published for open TAAA repair are attributed to Crawford’s, Coselli’s, and Safi’s groups out of Houston, Texas. Both Coselli and Safi trained in Houston at Baylor College of Medicine under Michael E. DeBakey and his protégé, E. Stanley Crawford, both of whom are considered preeminent pioneers in aortic surgery. Crawford’s entire experience reported in 1993 included results from more than 1500 TAAA repairs with a 30-day survival of 92%, a benchmark that many centers today struggle to match (28). The incidences of acute renal failure (ARF) and spinal cord ischemia (SCI) were 9.0% and 15.5%, respectively, and subsequent studies have improved greatly on the prevention of these complications. Coselli et al. published a series of nearly 2,300 consecutive TAAA repairs with an exemplary 95% 30-day survival and greatly reduced rates of ARF (5.6%) and SCI (3.8%). The drastic reduction in perioperative complication rates related to ARF and SCI are attributed to selective cold crystalloid perfusion of the kidneys and CSF drainage, respectively (29). Safi’s group reported similar results using the same adjuncts (30). Houston’s combined efforts have created a new standard for mortality and perioperative complication rates in modern open TAAA repair. A summary of these studies is included in Table 1. It is interesting to note that the highest survival published to date was also the largest series ever reported, indicating that high volume centers can achieve the best outcomes in this disease process. Outcomes related to both endovascular and hybrid repairs are extensively reviewed elsewhere in this issue.

Full table
Future perspective
When reviewing current outcomes of open TAAA repair strategies, it is clear that exemplary mortality rates are only achieved at select high-volume centers. Historically, this trend was also seen with open AAA repairs. With the emergence of total endovascular therapy as the new gold standard for AAA repair, it is reasonable to assume that with increased use and innovation of endovascular techniques, an era will arrive in which TAAAs may be more universally treated with less invasive techniques. Until commercially available devices are readily available and their use is easily replicated by a multitude of surgeons, however, open repair remains as the best option for the majority of patients to achieve good long-term results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Etheredge SN, Yee J, Smith JV, et al. Successful resection of a large aneurysm of the upper abdominal aorta and replacement with homograft. Surgery 1955;38:1071-81.
- Rob C. The surgery of the abdominal aorta and its major branches. Ann Roy Coll Surgeons, England 1955;17:307-17.
- DeBakey ME, Cooley DA. Successful resection of aneurysm of thoracic aorta and replacement by graft. J Am Med Assoc 1953;152:673-6.
- DeBakey ME, Creech O Jr, Morris GC Jr. Aneurysm of thoracoabdominal aorta involving the celiac, superior mesenteric, and renal arteries; report of four cases treated by resection and homograft replacement. Ann Surg 1956;144:549-73.
- DeBakey ME, Crawford ES, Garrett HE, et al. Surgical considerations in the treatment of aneurysms of the thoraco-abdominal aorta. Ann Surg 1965;162:650-62.
- Crawford ES. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg 1974;179:763-72.
- Huynh TTT, Estrera AL, Millers CC, et al. Thoracic vasculature (with emphasis on the thoracic aorta). In: Townsend CM, Beauchamp RD, Evers BM, et al. eds. Sabiston textbook of surgery: the biological basis of modern surgical practice. 17th ed. New York: Elsevier Saunders, 2004.
- Ito S, Akutsu K, Tamori Y, et al. Differences in atherosclerotic profiles between patients with thoracic and abdominal aortic aneurysms. Am J Cardiol 2008;101:696-9.
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8.
- Escobar GA, Upchurch GR Jr. Management of thoracoabdominal aortic aneurysms. Curr Probl Surg 2011;48:70-133.
- Juvonen T, Ergin MA, Galla JD, et al. Risk factors for rupture of chronic type B dissections. J Thorac Cardiovasc Surg 1999;117:776-86.
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994;107:1323-32; discussion 1332-3.
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998;280:1926-9.
- Juvonen T, Ergin MA, Galla JD, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg 1997;63:1533-45.
- Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg 2012;56:565-71.
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002;74:S1877-80; discussion S1892-8.
- Crawford ES, Coselli JS. Thoracoabdominal aneurysm surgery. Semin Thorac Cardiovasc Surg 1991;3:300-22.
- Safi HJ, Miller CC 3rd. Spinal cord protection in descending thoracic and thoracoabdominal aortic repair. Ann Thorac Surg 1999;67:1937-9; discussion 1953-8.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841-57.
- Elefteriades JA, Botta DM Jr. Indications for the treatment of thoracic aortic aneurysms. Surg Clin North Am 2009;89:845-67, ix.
- Schepens MA, Heijmen RH, Ranschaert W, et al. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5.
- Huynh TT, van Eps RG, Miller CC 3rd, et al. Glomerular filtration rate is superior to serum creatinine for prediction of mortality after thoracoabdominal aortic surgery. J Vasc Surg 2005;42:206-12.
- Coselli JS, LeMaire SA. Descending and Thoracoabdominal Aortic Aneurysms. In: Cohn LH. eds. Cardiac surgery in the adult. 3rd ed. New York: McGraw-Hill, 2008.
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8.
- Cinà CS, Abouzahr L, Arena GO, et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aortic aneurysm surgery: a systematic review and meta-analysis. J Vasc Surg 2004;40:36-44.
- Roselli EE, Greenberg RK, Pfaff K, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2007;133:1474-82.
- Kabbani LS, Criado E, Upchurch GR Jr, et al. Hybrid repair of aortic aneurysms involving the visceral and renal vessels. Ann Vasc Surg 2010;24:219-24.
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368-70.
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4; discussion S890-2.
- Estrera AL, Miller CC 3rd, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg 2005;80:1290-6; discussion 1296.





