Hypertrophic cardiomyopathy in children
Definition and morphology
Hypertrophic cardiomyopathy (HCM) is a primary genetic myocardial disease usually characterized by asymmetric ventricular septal hypertrophy. This literature review aims to provide an overview of HCM in children and to guide rational decision-making and surgical management of this highly specialized condition. The histological hallmark of HCM is myocardial fiber disarray consisting of short runs of severely hypertrophied fibers interspersed by connective tissue (i.e., interstitial fibrosis). The latter is an important histological feature that has been associated with arrhythmogenesis and risk of sudden cardiac death (1,2).
Although HCM is a common genetic disorder in adults (1/500) (3,4), it is rare in the pediatric population. Presentation in childhood is most often associated with dynamic left ventricular outflow tract (LVOT) obstruction and variable degrees of mitral valve regurgitation (MR). LVOT obstruction in HCM is a consequence of the exaggerated myocardial hypertrophy, combined with systolic anterior motion (SAM) of the anterior mitral leaflet and subvalvular apparatus. Meanwhile, MR occurs as a result of loss of MV leaflet coaptation caused by SAM. LVOT obstruction associated with HCM is distinct in both morphologic appearance and prognosis from congenital discrete membranous sub-aortic stenosis (5).
There are variable distributions of LV hypertrophy in patients with HCM. Classically, obstructive HCM in children is associated with prominent thickening of the anterior basal septum opposite to the anterior leaflet of the mitral valve (Figure 1). Other morphologies encountered include mid-septal hypertrophy, which typically results in both basal LVOT and mid-left ventricular obstruction (Figure 2), and apical hypertrophy, which is often associated with no obstruction, but rather, limitation of diastolic filling related to restricted LV cavitary volume (Figure 3).
Genetic basis
Sixty to eighty percent of the cases of HCM are familial and multiple genetic mutations have been associated with this disease (6). Most patients with familial disease have mutations in one of three genes, either β-myosin heavy chain (MYH7) in 35% to 50%; myosin-binding protein C (MYBPC3) in 15% to 25%; or cardiac troponin T type 2 (TNNT2) in 15% to 20%. Identification of the specific mutation with a family may aid in screening other at-risk family members, although the optimal screening strategy remains controversial.
Presentation and symptoms
The clinical features and course of HCM in children, as in adults, is highly variable, ranging from complete absence of symptoms to dyspnea/angina or even sudden death (7-10). Symptoms in children are often related to the severity of MR and/or diastolic myocardial dysfunction. Interestingly, although the severity of LVOTO and MR are usually similar, some patients may present with minimal or no SAM-mediated MR and conversely, there can be mild-to-moderate LVOT obstruction with severe SAM-mediated MR. While significant obstruction typically causes symptoms in adults, the correlation is less clear in childhood, either because the patients self-limit their activities or have improved compensation at a younger age. There are some symptomatic patients who do not have substantial LVOT obstruction. Symptoms in these cases are caused by a complex interaction of diastolic dysfunction, arrhythmias, myocardial ischemia, and MR. The usual findings on physical examination are related to LVOT obstruction and MR, and include a classic crescendo-decrescendo murmur best auscultated near the left sternal border in those with LVOTO. It peaks in mid to late systole and usually ends before the second heart sound. A bifid arterial pulse may be noted with a rapid upstroke, in contrast to the anacrotic pulse of aortic stenosis. A pansystolic murmur at the apex is heard when significant MR is present. The apical impulse may be multiple in those with LVOTO and abnormal diastolic filling. There may also be an audible 4th heart sound with significant LV hypertrophy. Infants and young children presenting with severe right-sided HOCM may be cyanotic from reversal of shunt flow at the atrial level.
It has been observed that progression of left ventricular hypertrophy may be more rapid in children and adolescents than in the adult population (11). Sudden death as an initial presentation is more common in the pediatric population (7,12). Symptomatic children with HCM have a higher death rate than do symptomatic adults, with annual mortality as high as 6% in pediatric cohorts (7,8,12). In contrast, the incidence of sudden cardiac death in adults with HCM has been reported around 1–3% (13-15).
Workup and diagnosis
Electrocardiogram characteristically shows LV hypertrophy with a strain pattern. Q waves and evidence of left atrial enlargement may be present. Occasionally, the ECG shows left bundle branch block and less frequently, right bundle branch block. Chest radiography shows evidence of LV hypertrophy and mild to moderate cardiomegaly, more often in HCM than in other forms of aortic outflow obstruction. Pulmonary venous congestion and enlargement of the pulmonary artery may be present.
Two-dimensional and Doppler echocardiography is the primary tool for the diagnosis of HCM. Echocardiography provides information on ventricular morphology, hemodynamics, systolic and diastolic function, as well as valve function. Provocative maneuvers (e.g., Valsalva) are useful in uncovering latent obstruction that may benefit from treatment. Echocardiography is also a useful tool to differentiate HCM from the so-called ‘athletic heart’. Patients with HCM, unlike athletic heart, have small LV cavity dimensions, as well as evidence of diastolic (reduced tissue Doppler velocity) and myocardial deformation (strain) abnormalities. These clinical features, along with the presence of a family history of HCM and/or reduced maximum oxygen consumption capacity help differentiate hypertrophy related to HCM from that associated with athletic training. In some instances, a period of detraining may be needed with repeat echocardiography that typically shows regression of hypertrophy in the athlete’s heart, which does not occur in patients with HCM.
In children with HCM, LV thickness can also change with time. For example, dramatic increases in LV mural thickness can develop during adolescence in those who initially showed minimal hypertrophy. This phenomenon has implications for the strategies used in echocardiographic screening of children of family members with known HCM, and underscores the importance of serial studies and genetic testing.
Magnetic resonance imaging can provide additional key information about the myocardium. It may identify regions of LV hypertrophy in those with limited acoustic windows. The most important application is the detection and grading of the severity of myocardial fibrosis, which appears to be an important risk factor for subsequent ventricular arrhythmias and sudden cardiac death in both adults and children (1,2,16).
In making the diagnosis of HCM, alternative causes of left ventricular hypertrophy, such as systemic hypertension, metabolic/storage disorders or aortic stenosis, must also be excluded.
Management
Non-surgical
Pharmacologic therapy, usually with beta-blockers and occasionally calcium channel blockers, represents the initial treatment strategy for symptomatic obstructive HCM. When generous doses are required, monitoring for side effects is critical. These may include depression and impaired growth, development, and performance at school. Other medications that may be employed include disopyramide, mexiletine, and amiodarone, depending on the presence of obstruction or arrhythmia. Medical therapy is often unsuccessful in reducing the resting gradient across the LVOT. In these cases, the aim of medical therapy is to abolish the catecholamine-induced effects that may exacerbate LVOT obstruction, minimize dysrhythmia and to decrease the heart rate, which allows more time for diastolic filling (7). There is no evidence that medical therapy reduces ventricular hypertrophy. Furthermore, vasodilators. diuretics, and inotropic agents such as digoxin should be generally avoided in these patients or used with caution.
Multiple approaches to medical therapy in asymptomatic patients with HCM have been advocated. Beta blockade is often used to blunt the catecholamine-mediated changes associated with exertion. The threshold is lower for treating patients at higher risk of adverse events, such as young age at presentation, a family history of sudden death, extreme septal hypertrophy, history of syncope, history of sustained or nonsustained ventricular tachycardia, abnormal blood pressure response with exercise, presence of congestive heart failure, and lower left ventricular fractional shortening (7,15,17). These patients should be considered for medical management even in the absence of symptoms. They should also be considered for implantable cardiac defibrillator (ICD) placement to reduce the risk of sudden cardiac death. In a study of 173 patients who were taking amiodarone, beta-blockers, verapamil, or sotalol for treatment of symptoms, there was no difference in sudden death mortality compared with patients who were receiving no pharmacologic therapy (18). Thus, ICD placement remains the primary therapy for prevention of sudden death in high-risk HCM patients.
Importantly, while alcohol septal ablation is an alternative septal reduction therapy in selected adults who are not considered candidates for surgery, alcohol ablation is not indicated in children and young adults (7).
Surgical
Extended left ventricular septal myectomy is the gold standard treatment for HCM in patients who are unresponsive to medical therapy (19,20). Based on the pattern of septal hypertrophy and physiologic consequences, the patient with HCM can be broadly categorized into three groups:
- The first group has typical subaortic septal hypertrophy with significant LVOT obstruction and SAM with or without significant mitral regurgitation. These patients are best served with transaortic septal myectomy, which will be described below. This group has been studied the most and has the largest body of published data. Successful transaortic septal myectomy in these patients will achieve low single digit LVOT gradient, resolution of SAM and mitral regurgitation (21).
- The second group has midventricular obstruction with or without an apical ‘pouch’ or aneurysm. These patients may or may not have SAM or chordal SAM. The transaortic approach may be challenging in this group of patients, particularly in children with a smaller aorta. In this setting, midventricular resection can be accomplished via apical left ventriculotomy with or without transaortic approach. We have previously reported our results of transapical approach to septal myectomy in this group of patients with comparable results to those achieved with the transaortic approach (22,23). Often determination of the distance from the aortic annulus to the point of maximal obstruction and comparison of that length to the distance from the apex to the obstruction and the diameter of the annulus itself can assist in surgical planning for a trans-aortic versus a trans-apical approach.
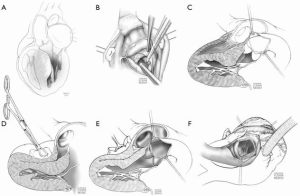
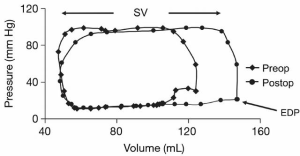
- The third group comprises patients without obstruction who suffer mainly from diastolic dysfunction or a small left ventricular cavity secondary to massive hypertrophy—usually in the apical septum. These patients can also develop an apical aneurysm (apical pouch) likely from subendocardial ischemia related to increased LV wall stress (24). Transapical myectomy with the intent of enlarging the LV cavity (when stroke volume is below the normal range) can postpone the need for heart transplant and can be considered in high volume experienced centers. It should be emphasized that only patients with reduced stroke volume will benefit from this procedure. These patients require meticulous preoperative selection with multiple imaging modalities and accurate measurements of LV stroke volume via MRI, CT and or contrast ventriculogram should be obtained. When carefully selected, these patients can have increases in their stroke volume with a lower left ventricular end diastolic pressure and improvement in their symptoms (Figure 4) (25).
In patients with LVOT obstruction, septal myectomy is the preferred treatment when the resting or provoked gradient is greater than 50 mmHg (7,26). Surgery may also be advised in children who are asymptomatic or mildly symptomatic with high gradients of between 75 and 100 mmHg at rest, or when there is severe concomitant mitral regurgitation (7). At present, there are a limited number of surgeons who have significant experience with septal myectomy. Indeed, this is not a procedure to be performed occasionally, as it is a difficult operation to perform well. That is, achieving the following is difficult: <1% early mortality, consistent reduction in gradient to <10 mmHg, <2% (low) complication rate for permanent pacemaker, <1% iatrogenic ventricular septal defect, avoidance of aortic or mitral valve injury (estimated to occur in 5% of cases), and comfort with dealing with the commonly associated mitral valvar and subvalvular abnormalities. Although the learning curve for septal myectomy is challenging, Iacovoni et al. demonstrate the technique of septal myectomy can be mastered if one is provided with sufficient case volume and focused expertise (27).
Surgical treatment of HCM was introduced by Kirklin from Mayo Clinic in 1958 with a simple myotomy without actual muscle resection (28). Surgical management further evolved and subsequently has been largely replaced by the more predictable and complete transaortic myectomy advocated by Morrow (29). This is the basis of the extended septal myectomy which we favor and describe here [initially described by Messmer (30)]. While the early surgical experience was associated with higher incidence of complications such as complete heart block, iatrogenic ventricular septal defect, injury to aortic and mitral valves and incomplete relief of obstruction, these are very uncommon in the current era.
Technique of operation
Transaortic approach for basal obstruction
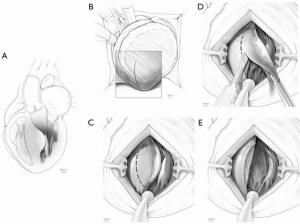
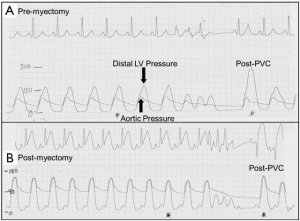
Surgery is done through a standard median sternotomy, and intracardiac pressures are measured directly using needles in the left ventricle and aorta prior to and after the myectomy (Figure 5). If the measured initial gradient is less than 30 mmHg because of the conditions of anesthesia, provocation with isoproterenol is performed to determine the maximum gradient. Intraoperative transesophageal echocardiography (TEE) is used routinely. Cardiopulmonary bypass is established and the aorta is cross-clamped followed by antegrade cold blood cardioplegia. The left heart is vented with a catheter inserted through the right superior pulmonary vein. An oblique aortotomy toward the middle of the noncoronary sinus is performed. A vein retractor is used to retract the right sinus of Valsalva and a suction catheter is used to retract and protect the mitral valve; care is taken to avoid retraction injury to the right coronary cusp. A hook retractor is used to engage the distal septum. The septal incision is begun using a number 10 scalpel blade at the base/nadir of the right aortic sinus, and continued leftward towards the mitral valve. Importantly, the incision is carried apically beyond the point of contact of the mitral valve leaflet with the septum, usually marked by a fibrous contact lesion. The resection is extended leftward toward the hinge of the mitral valve, and apically to the bases of the papillary muscles. Resection of the apical third of the septum to the right of the incision in the aortic sinus is then performed, effectively making a much wider trough at the midventricular level. The myectomy trough should be opposite the anterior mitral leaflet, the subvalvular apparatus and the papillary muscles; it should be widest at the midventricular level and apical extent of the trough is most important. The most common mistake early in a surgeon’s learning curve is not extending the myectomy trough far enough (apically) into the ventricle thus converting basal obstruction to midventricular obstruction.
The methods of extended myectomy as described can be more difficult in children, when the orifice of the aortic valve is small. This results in poor exposure of the septum, inability to perform a complete myectomy and ultimately, a greater likelihood of residual obstruction. In addition, there is more likelihood of injury to the mitral and aortic valves. For all of these reasons, minimally invasive techniques (for example, mini-sternotomy or robotics) are not used routinely in this setting, particularly in children. The most common reason for residual/recurrent obstruction is incomplete extension of the myectomy far enough down into the left ventricle (Figure 1C). A sponge stick can be used to depress the right ventricle and to rotate the septum posteriorly, orienting the LV outflow anteriorly and allowing better exposure of the distal extent of the myectomy (Figure 1D). After separation from bypass, pressures are measured again in the left ventricle and aorta and TEE is repeated. If successful myectomy has been performed, there will be little or no residual gradient, and little or no SAM of the anterior leaflet of the mitral valve. The improved systolic MV geometry eliminates the MR in the majority without the need for any direct manipulation of the valve itself. In general, bypass would be resumed for re-resection if the measured peak-to-peak gradient is 15 to 20 mmHg, or if there is persistent SAM of the anterior leaflet of the mitral valve. In general, chordal SAM does not result in significant residual obstruction across the outflow tract.
Transapical approach for midventricular obstruction
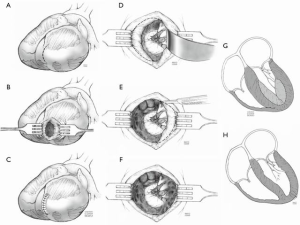
This approach is applied for midventricular obstruction without SAM; by definition, the obstruction is too far from the aortic valve to safely reach from a transaortic approach. After arresting the heart, a moist laparotomy pad is placed in the pericardial well behind the LV, and the apex of the heart is delivered anteriorly. The left anterior descending artery (LAD) is identified, and an apical ventriculotomy, approximately 1.5–2 cm in length is made 1–1.5 cm lateral to it (Figure 2). This incision is located over the apical dimple (when one is present) and should be situated far enough to the left of the LAD to allow secure closure of the ventriculotomy without compromise of the vessel. For patients with midventricular obstruction, there will usually be an adequate apical chamber and in some circumstances, there may be an “apical pouch” that is subsequently obliterated with ventriculotomy closure. Commonly, endocardial scar is seen on the septum in the area of apposition with the papillary muscles, which makes contact with the septum during systole and resulting in obstruction. Myectomy begins in the septum with removal of the endocardial scar and muscle below and above. If the papillary muscles are greatly enlarged, these can be shaved as well in order to eliminate midventricular apposition.
Transapical approach for LV cavity enlargement
This approach is applied for patients with a small left ventricular cavity, i.e., less than normal stroke volume. The operation is started, the heart arrested and ventriculotomy is performed as describe above (Figure 3). The apex of the heart is usually obliterated with muscle and initial myectomy is performed along the septum with particular attention to avoid inadvertent injury to the apically displaced papillary muscles. After identification of the anterolateral and posteromedial papillary muscles, myectomy is extended to the ventricular free walls with the goal of enlarging the apical third of the LV cavity. The ventricular cavity is also augmented by means of excision of hypertrophied trabeculae and by careful shaving of the hypertrophied papillary muscles. The resection removes the mass of myocardium filling the apex of the heart. Importantly, care is taken to avoid resecting too much muscle at the edges of the ventriculotomy (which can compromise closure and result in apical aneurysm). The apical ventriculotomy is closed in 2 layers over strips of felt (25). Data from our initial series was confined to adult patients (25); this approach has also been performed in children in our practice as a bridge to transplant and data will be forthcoming.
Outcome
Risk of hospital death after isolated septal myectomy for obstructive HCM in adults is low [less than 1% in experienced centers (31)]. Although the operation is technically more challenging in children because of difficulty of exposure of the smaller structures, there is a role for surgery in the pediatric group with similarly very low risk and good results. This is particularly true for basal outflow tract obstructions. Between 1975 and 2010, 127 consecutive patients at Mayo Clinic, ranging from 2 months to 21 years old were recently reported (32). All patients had extended myectomy performed with no in-hospital mortality. Concomitant procedures included resection/division of accessory papillary muscle(s) or attachments in 17 patients, mitral repair in 12 (2 for iatrogenic injury and 10 for redundant leaflets), closure of patent foramen ovale or atrial septal defect in seven patients, and aortic valve repair in seven (iatrogenic injury). Hemodynamic measurements and echocardiographic data demonstrated mean preoperative septal wall thickness of 24.4±9.4 mm and posterior free wall thickness of 13.6±4.4 mm. The mean preoperative gradient was 90 mmHg which was reduced to 6 mmHg postoperatively (P<0.0001). Intraoperative assessment of the mitral valve demonstrated a significant reduction of mean echocardiographic regurgitation grade from 2 to 1 (P<0.0001). There were no early deaths. There was one iatrogenic ventricular septal defect and one patient required a permanent pacemaker for complete heart block that had resolved at a year follow up.
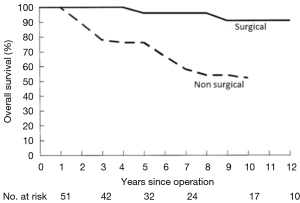
Clinical follow-up was achieved with a mean period of 8.3±8.2 years (median 5.3, maximum 37 years). Overall survival was 99%, 95%, and 92% at 5, 10, and 20 years, respectively. There were four late deaths; one sudden death in a patient with documented relief of obstruction on follow up. Two patients died from rejection after heart transplantation, which had been performed 3 and 6 years after myectomy due to progressive restrictive physiology and diastolic heart failure. One patient died from unknown causes. Six patients underwent repeat septal myectomy (an average 6 years after initial myectomy); two required AVR and two had MVR. At last follow-up, 96% of patients were in New York Heart Association class I or II. Late survivorship compared very favorably with the natural history of the disease (Figure 6), for which there is an annual mortality rate as high as 6% in symptomatic patients evaluated in tertiary referral centers (7).
Surgical myectomy does not eliminate the need to assess the risk of each patient for sudden cardiac death. Nor does it eliminate the need for consideration for ICD in the high-risk patient groups discussed before. In a recent large cohort study of young, high-risk HCM patients, low mortality rates were achieved with the application of contemporary cardiovascular treatment strategies, largely because of reliable identification of high-risk patients who benefited from ICD for sudden death prevention, thereby creating the opportunity for extended longevity and a good quality of life (34).
We acknowledge the limitations of this paper, as a review article that reports the available literature can be influenced by the biases of the author’s personal viewpoints and experience, and potential gaps in literature searching or errors in the translation of data from the primary literature to summarization in the review. Nonetheless, it summarizes the world’s largest institutional experience of HCM in children in a concise and practical format.
We conclude that extended septal myectomy can be performed in symptomatic pediatric patients with obstructive HCM with very low mortality and excellent relief of symptoms. Early significant reductions in LVOT obstruction and degree of mitral regurgitation are maintained at late follow-up in the majority of patients. Late survivorship after myectomy is improved compared to the natural history of this disease without surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Moon JC, McKenna WJ, McCrohon JA, et al. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003;41:1561-7. [Crossref] [PubMed]
- Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51:1369-74. [Crossref] [PubMed]
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995;92:785-9. [Crossref] [PubMed]
- Maron BJ, Spirito P, Roman MJ, et al. Prevalence of hypertrophic cardiomyopathy in a population-based sample of American Indians aged 51 to 77 years (the Strong Heart Study). Am J Cardiol 2004;93:1510-4. [Crossref] [PubMed]
- van Son JA, Schaff HV, Danielson GK, et al. Surgical treatment of discrete and tunnel subaortic stenosis. Late survival and risk of reoperation. Circulation 1993;88:II159-69. [PubMed]
- Marian AJ, Salek L, Lutucuta S. Molecular genetics and pathogenesis of hypertrophic cardiomyopathy. Minerva Med 2001;92:435-51. [PubMed]
- American College of Cardiology Foundation/American Heart Association Task Force on Practice G. American Association for Thoracic S, American Society of E, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2011;142:1303-38. [Crossref] [PubMed]
- Maron BJ, Henry WL, Clark CE, et al. Asymetric septal hypertrophy in childhood. Circulation 1976;53:9-19. [Crossref] [PubMed]
- McKenna W, Deanfield J, Faruqui A, et al. Prognosis in hypertrophic cardiomyopathy: role of age and clinical, electrocardiographic and hemodynamic features. Am J Cardiol 1981;47:532-8. [Crossref] [PubMed]
- Maron BJ, Tajik AJ, Ruttenberg HD, et al. Hypertrophic cardiomyopathy in infants: clinical features and natural history. Circulation 1982;65:7-17. [Crossref] [PubMed]
- Maron BJ, Spirito P, Wesley Y, et al. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N Engl J Med 1986;315:610-4. [Crossref] [PubMed]
- Maron BJ, Roberts WC, Edwards JE, et al. Sudden death in patients with hypertrophic cardiomyopathy: characterization of 26 patients with functional limitation. Am J Cardiol 1978;41:803-10. [Crossref] [PubMed]
- Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102:858-64. [Crossref] [PubMed]
- Kofflard MJ, Waldstein DJ, Vos J, et al. Prognosis in hypertrophic cardiomyopathy observed in a large clinic population. Am J Cardiol 1993;72:939-43. [Crossref] [PubMed]
- Lipshultz SE, Orav EJ, Wilkinson JD, et al. Risk stratification at diagnosis for children with hypertrophic cardiomyopathy: an analysis of data from the Pediatric Cardiomyopathy Registry. Lancet 2013;382:1889-97. [Crossref] [PubMed]
- Chaowu Y, Shihua Z, Jian L, et al. Cardiovascular magnetic resonance characteristics in children with hypertrophic cardiomyopathy. Circ Heart Fail 2013;6:1013-20. [Crossref] [PubMed]
- Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342:1778-85. [Crossref] [PubMed]
- Melacini P, Maron BJ, Bobbo F, et al. Evidence that pharmacological strategies lack efficacy for the prevention of sudden death in hypertrophic cardiomyopathy. Heart 2007;93:708-10. [Crossref] [PubMed]
- Nishimura RA, Giuliani E, Brandenburg R, et al. Hypertrophic cardiomyopathy. In: Gersh BJ, McGoon MD, Hayes D, et al. editors. Mayo Clinic Practice of Cardiology. 3rd ed. St. Louis, MO: Mosby-Year Book Inc., 1996:689-711.
- Theodoro DA, Danielson GK, Feldt RH, et al. Hypertrophic obstructive cardiomyopathy in pediatric patients: results of surgical treatment. J Thorac Cardiovasc Surg 1996;112:1589-97; discussion 1597-9. [Crossref] [PubMed]
- Dearani JA, Ommen SR, Gersh BJ, et al. Surgery insight: Septal myectomy for obstructive hypertrophic cardiomyopathy--the Mayo Clinic experience. Nat Clin Pract Cardiovasc Med 2007;4:503-12. [Crossref] [PubMed]
- Kunkala MR, Schaff HV, Nishimura RA, et al. Transapical approach to myectomy for midventricular obstruction in hypertrophic cardiomyopathy. Ann Thorac Surg 2013;96:564-70. [Crossref] [PubMed]
- Said SM, Schaff HV, Abel MD, et al. Transapical approach for apical myectomy and relief of midventricular obstruction in hypertrophic cardiomyopathy. J Card Surg 2012;27:443-8. [Crossref] [PubMed]
- Goel K, Schaff HV, Nishimura RA. Natural history of apical hypertrophic cardiomyopathy and novel surgical treatment. J Thorac Cardiovasc Surg 2016;152:626-7. [Crossref] [PubMed]
- Schaff HV, Brown ML, Dearani JA, et al. Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2010;139:634-40. [Crossref] [PubMed]
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287:1308-20. [Crossref] [PubMed]
- Iacovoni A, Spirito P, Simon C, et al. A contemporary European experience with surgical septal myectomy in hypertrophic cardiomyopathy. Eur Heart J 2012;33:2080-7. [Crossref] [PubMed]
- Kirklin JW, Ellis FH Jr. Surgical relief of diffuse subvalvular aortic stenosis. Circulation 1961;24:739-42. [Crossref] [PubMed]
- Morrow AG. Hypertrophic subaortic stenosis. Operative methods utilized to relieve left ventricular outflow obstruction. J Thorac Cardiovasc Surg 1978;76:423-30. [PubMed]
- Messmer BJ. Extended myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg 1994;58:575-7. [Crossref] [PubMed]
- Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 2007;116:196-206; discussion 206. [Crossref] [PubMed]
- Altarabsheh SE, Dearani JA, Burkhart HM, et al. Outcome of septal myectomy for obstructive hypertrophic cardiomyopathy in children and young adults. Ann Thorac Surg 2013;95:663-9; discussion 669. [Crossref] [PubMed]
- Minakata K, Dearani JA, O'Leary PW, et al. Septal myectomy for obstructive hypertrophic cardiomyopathy in pediatric patients: early and late results. Ann Thorac Surg 2005;80:1424-9; discussion 1429-30. [Crossref] [PubMed]
- Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic Cardiomyopathy in Children, Adolescents, and Young Adults Associated With Low Cardiovascular Mortality With Contemporary Management Strategies. Circulation 2016;133:62-73. [Crossref] [PubMed]