Coronary artery bypass grafting (CABG) vs. percutaneous coronary intervention (PCI) in the treatment of multivessel coronary disease: quo vadis? —a review of the evidences on coronary artery disease
Introduction
The optimal treatment of ischemic coronary artery disease (CAD) is still at the center of a heated debate. A number of randomized trials (RCTs) and a plethora of retrospective studies investigated the outcomes and risk/benefit balance of the two accepted approaches in CAD, namely percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) surgery. These trials have inspired the current guidelines, despite the presence of some unsolved questions (1). In this context, efforts have been undertaken in order to elucidate the best treatment for unprotected left main (LM) disease, and up-to-date meta-analyses of RCTs have been performed with this aim in mind (2-6). If the general consensus for LM disease seems to be trending towards the equivalence of PCI and CABG with regards to safety outcomes, with an increase in repeat revascularization in PCI and a marginal increase in strokes in CABG (7-11), in the more common situation of multivessel disease (MVD) an acceptable level of agreement has not been reached yet. Several confounding factors inherent to the nature of both RCT and meta-analyses (i.e., statistical underpower, selection bias, inclusion of both LM disease and MVD, etc.) could have conspired against the possibility to reach an unbiased conclusion. The difficulty in generalizing the results to the real-life scenario of CAD might have played an additional role. Moreover, the currently published systematic reviews cumulating the results of the available RCTs are often in disagreement, preventing a definitive analysis of the evidence on the topic at present. Therefore, we reviewed the current literature and the available meta-analyses data on the comparison between PCI and CABG focusing on MVD only.
The clinical trials
Since the advent of drug-eluting stents (DES) and the evidence attesting to their superiority over bare metal stents (12), several trials have been published investigating PCI outcomes in comparison to CABG. The BEST investigators undertook an RCT to demonstrate non-inferiority of Everolimus eluting stent in respect to CABG. Despite being abandoned due to slow recruitment, the trials produced results from more than 800 patients, demonstrating an occurrence of the primary endpoint, a composite of death due to myocardial infarction (MI) or target-vessel revascularization at 2 years, of 11.0% in the patients in the PCI group and of 7.9% in those in the CABG group with a still significant difference at longer follow-up [median, 4.6 years (15.3% of the patients in the PCI group and in 10.6% of those in the CABG group)] (13).
The SYNTAX trial tested non-inferiority of PCI versus CABG in 1,800 patients. Non-inferiority criteria were not met as rates of major adverse cardiac or cerebrovascular events at 12 months were significantly higher in the PCI group (17.8%, vs. 12.4% for CABG). This was thought to be due to an increased rate of repeat revascularization (13.5% vs. 5.9%) in the PCI group (14,15). The CARDia trial was the first examining the treatment of CAD in a subgroup of diabetic patients, demonstrating the superiority of CABG in this subset with combined rates of mortality, MI, stroke and repeated revascularization of 11.3% in the CABG group and 19.3% in the PCI group at 1 year (16). The FREEDOM trial confirmed these findings in 1,900 patients with complex MVD and diabetes, demonstrating comparatively worse 5-year rates of a composite outcome, including death from any cause, nonfatal MI, or nonfatal stroke, in the PCI group (26.6% vs. 18.7% in the CABG group). Despite the incidence of stroke being higher in CABG cohort, death and MI were significantly higher in the PCI group, leading to the conclusion that diabetic population would best benefit from CABG rather than PCI (17).
Subsequently, the VA-CARDS investigators reported the results of a randomized trial comparing interventions exclusively with drug-eluting stents and surgery in patients with diabetes and high-complex CAD. Despite being underpowered, all-cause mortality was 5.0% for CABG and 21% for PCI at 2 years follow-up, while the risk for nonfatal MI was 15% for CABG and 6.2% for PCI (18) (Table 1).
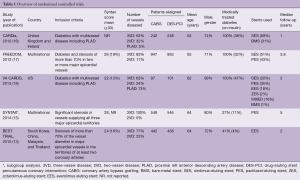
Full table
The meta-analyses
Despite the significance of the conclusion reached by the aforementioned RCTs, several meta-analyses have been performed with the aim of circumventing the issues pertaining to being under-powered.
Our group performed a systematic review and meta-analysis of five RCTs, encompassing a total of 4,563 patients with MVD (19). PCI with DES was associated with a 154% increased relative risk of repeat revascularization, late mortality (increased by 51%) and MI (increased by 102%) when compared to CABG. On the hand, CABG was hampered by a 29% increase risk of stroke, but the absolute risk increase in stroke was minimal when compared with the absolute risk reduction in mortality and MI. A number-to-treat analysis demonstrated that placing CABG over DES-PCI in 100 subjects, 3–4 deaths, 4–5 MI and 8–9 repeat revascularizations would be prevented at the expense of one extra stroke after an average follow-up of 3.4 years. Interestingly, subgroup analysis suggested that CABG would improve survival and minimize the risk of subsequent MI independent of the presence of diabetes. Conversely, the increased risk of stroke associated with CABG might be of clinical significance only in diabetic patients.
Moreover, the results of this meta-analysis refuted the existing dogmatic view that the survival benefit guaranteed by CABG is only relevant to three-vessel disease. Conversely, the benefit of CABG was also significant in the presence of two vessel disease and/or proximal left descending artery disease. On the other hand, data showed that the increased risk of subsequent MI following DES-PCI was likely to be significant only in cases of three-vessel disease (19).
Another large meta-analysis encompassing seven RCTs for a total 5,835 patients confirmed the results of the previously mentioned study, demonstrating a reduction in the mortality risk, MI and repeated revascularization in CABG versus first generation DES, at the expense of an increased stroke risk (20).
Sipahi and colleagues performed another review including six randomized studies (N=6,055), with their meta-analysis illustrating a significant reduction in total mortality, MI, and repeat revascularization with CABG compared with PCI (21). However, unlike the previous studies, these authors found a trend toward excess strokes with CABG, but this was not significant. The conclusions drawn suggest CABG as the best treatment option in patients with MVD compared with PCI, given the undisputable reduction in long-term mortality, MIs and repeat revascularizations, irrespective of the presence of diabetes (21).
An interesting work has been recently reported by Fanari and colleagues, who performed a meta-analysis of six RCTs and investigated the results of the long-term follow-up of the studies. Despite potential bias due to the presence of an additional RCT involving unprotected LM disease (22), this study demonstrated that at 1 year, PCI was associated with a significantly higher incidence of target vessel revascularization, lower incidence of stroke and no difference in death or MI compared to CABG. However, at 5 years, PCI was associated with a higher incidence of death and MI. Increased mortality in the PCI group was mainly found in diabetics (23).
An ad hoc meta-analysis on MVD in the diabetic population encompassing 14 studies (five RCTs and nine observational) documented a much higher risk of repeat intervention and adverse cardiovascular/cerebrovascular events in the DES/PCI cohort compared to CABG, although early morbidities seemed to favor percutaneous procedures (24).
A previous and similar study on diabetic population confirmed that CABG in diabetic patients with MVD at low to intermediate surgical risk (defined as EUROSCORE <5) is superior to MVD PCI with DES. Despite an increase in stroke risk, CABG reduced overall death, nonfatal MI, and repeat revascularization (25).
In a meta-regression analysis using event rates as a dependent variable to test for an interaction between baseline clinical features (i.e., age, gender, diabetes mellitus, previous MI and ejection fraction) and choice of revascularization, D’Ascenzo et al. concluded that PCI significantly reduces the risk of stroke compared to CABG. particularly in female patients, but the risk of revascularization is increased with PCI, especially in women and in those with diabetes (26).
An interesting point has been raised in meta-analyses and systematic reviews on CAD with respect to the comparison of outcomes in complete or incomplete revascularization. A large preliminary investigation, including 35 studies and 89,883 patients, demonstrated that complete revascularization is more commonly achieved with CABG rather than PCI, and that incompleteness of revascularization is associated to increased mortality and repeated revascularization independently on the mode of treatment (27). Zimarino and colleagues echoed those results in another meta-analysis of 28 studies reporting on clinical outcomes of MVD patients treated with complete and incomplete revascularization, with extensive (>80%) use of stents for PCI or arterial conduits in CABG. They achieved similar results and demonstrated a larger clinical benefit of complete revascularization in diabetic patients. Interestingly, the survival benefit and reduction in relative risk of cardiovascular events was better in the patients enrolled in the more recent studies (28).
More recently, Lee et al. in a meta-analysis of 3,280 patients pooled results from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease), PRECOMBAT (Premier of Randomized Comparison of Bypass Surgery vs. Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease), and SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) trials. The study was focused on a composite outcome including of all-cause death, MI, or stroke.
The results showed that CABG, as compared with PCI with DES, decreased long-term rates of the composite outcome and repeat revascularization, when compared with DES-PCI in LM or MVD, although the advantage of CABG was more pronounced in the latter subcategory (10).
Chang et al. reported the results of a patient-level meta-analysis comparing the effect of CABG versus PCI with DES on long-term mortality in 1,275 nondiabetic patients with multivessel CAD. Results showed superiority of CABG in terms of short and long-term mortality, MI and repeat revascularization, in absence of significant group differences as far as stroke was concerned. The conclusions drawn echoed previous results and demonstrated superiority of CABG also in nondiabetic patients with multivessel CAD (29).
In a pooled analysis of individual patient-level data of the SYNTAX and BEST randomized trials, Cavalcante et al. analyzed the outcomes of 1,166 patients in which 577 were randomized to PCI and 589 to CABG. In patients with MVD with proximal left anterior descending artery (LAD) involvement, CABG is associated with a significantly lower rate of cardiac death, MI and all-cause revascularization when compared with DES-PCI. There was no difference among the groups as far as all-cause mortality and stroke were concerned, but the combined outcome of major adverse cardiovascular and cerebrovascular events (i.e., all-cause death, MI, stroke, revascularization) favored CABG. The authors concluded that in patients with MVD CABG was superior in terms of survival and cardiovascular events to drug-eluting stents at 5 years of follow-up (30).
Nevertheless, a recent systematic review featured in The Lancet by Head and colleagues, including 11 randomized trials and involving a total of 11,518 patients, illustrated equivalence in the long-term safety outcomes between the modalities of revascularization for unprotected LM disease. Conversely, the benefit of CABG was restricted to complex MVD and diabetic patients (31) (Table 2).
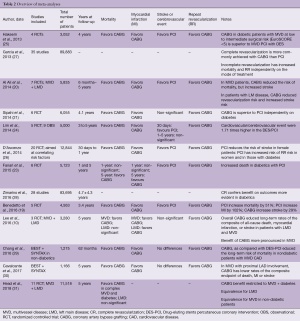
Full table
Discussion
The optimal treatment strategy for CAD remains controversial, as illustrated by the contradictory conclusions reached even in the context of meta-analyses of the same RCTs. Although some standpoints, such as the higher risk of repeat revascularization and cardiovascular event in DES-PCI remain consistent throughout the literature, the hard endpoints regarding early mortality, long-term survival and strokes are constantly put in doubt by subsequent study sub-analyses. The reason underlying this uncertainty might find its root in the low power and statistical bias inherent in some of the studies included in the systematic reviews.
The mortality rate of treated CAD has dramatically reduced over the years, independently of the mode of revascularization adopted. Consequently, conspicuous sample sizes are required to achieve significance, given the diminishing power of these studies. On one side, this calls for new event-driven designed trials, and on the other, it makes the interpretation and reliability of the results reached by both RCTs and their systematic reviews/meta-analyses rather difficult. The composite primary endpoint of major cerebrovascular and cardiac adverse events (MACCE) recurrently described in every RCT represents a product of this issue, and has been introduced to avoid the power limitations of these trials.
From the review of both the trials and meta-analyses, it appears that the main pillar sustaining the benefit of CABG compared to PCI is the reduced rate of repeat revascularizations, as the weight of the lower rate of MACCEs is jeopardized by the relative increased incidence of stroke in this group. However, repeat revascularization is considered a “soft” endpoint and no trial seems to have been adequately powered to assess the more important endpoint of mortality (32). Even the most recent study by Head et al. (31) with a sample size of more than 11,500 patients, despite confirming the superiority of CABG for MVD in diabetic patients, is not in agreement with the meta-analyses published immediately prior by Chang et al. (29) and Cavalcante et al. (30).
An interesting point that is emerging from the analysis of the literature is that of the issue of incomplete revascularization in the two modes of revascularization. Very recently, Hannan et al. reported the results of an interesting registry analysis on the outcomes of incomplete revascularization in PCI in a very large cohort of patients (33). The results of the study somehow echo the conclusion of the most recent follow-up of the SYNTAX study by Milojevic et al., in which authors demonstrated that incomplete revascularization was an independent predictor of mortality in the PCI group. Interestingly, in the SYNTAX trial, incomplete revascularization did not increase the risk of death or cardiac adverse events in the CABG arm of the study (34). These results reflect the findings of an analysis of the NICOR database including 13,701 patients who underwent CABG. After propensity score matching, incomplete revascularization did not increase all-cause death in the group (35). The mechanisms underlying this apparent inefficacy of PCI in incomplete revascularization still remains unsolved. Chronic total occlusion—which is difficult to manage percutaneously—and post-procedural MI exacerbate the already incomplete revascularization, have been advocated as culprit factors in determining mortality in PCI (36).
These events might similarly occur in surgical settings, however, they do not constitute a significant risk factor for CABG patients. From the BARI trial, we learnt that grafting left internal mammary artery (LIMA) to LAD determines survival independently of the presence of other grafts and that it seems that there is no direct numeric correlation between grafts and coronary lesions to achieve clinical benefit (37). On the other hand, arterial grafts have been shown to release high quantities of nitric oxide (NO), a known inducer of angiogenesis, and this is thought to be one of the factors at the crux of the superior outcomes of these conduits in CABG (38). It has been hypothesized that intramyocardial delivery of NO through the graft, together with the neoangiogenic drive initiated by the reperfusion, may account for the creation of a progressively spreading microvascular network of neocapillaries within the affected myocardium. These territorially expand from the region directly subjected to revascularization to the adjacent territories, therefore restoring the function of ungrafted regions (39).
This hypothesis finds a clinical correlate in the analysis of the NICOR registry, in which large territories that are tributaries of the right coronary artery or the circumflex artery remain unrevascularized leading to a reduction in late survival (35). More ad hoc studies are required to elucidate the pathophysiological mechanisms and the consequent optimal strategy to be adopted in these circumstances.
Another point of discussion centers on the fact that the currently available RCTs are comparing the newest generation of stenting technology with a relatively “old-fashioned” operation; the patency rate and durability of venous grafts is widely known to be limited. On account of the evidence testifying to the longer-term durability of CABG performed with a total arterial technique, a more adequate comparison would be performed among the newest stenting technology and more modern grafting strategies (i.e., total arterial revascularization).
This point is even more significant when noting that the majority of the trials and reviews, despite showing non-inferiority of PCI with respect to CABG for the safety endpoints in the immediate postoperative period, fail to demonstrate a sustained benefit of percutaneous interventions over the long-term. CABG seems to outperform PCI in the long-term with conduits known to have a limited life. We could imagine that even more compelling results would arise from the comparison of the long-term data of multiple arterial grafting trials, such as the ART trial (40), with the long-term durability of the newest generation DES.
Summarizing the current evidence from the largest RCTs, SYNTAX demonstrated superiority of CABG in cases of SYNTAX scores >22 (15). The BEST (13) and FREEDOM (17) trials showed the same superiority, irrespective of the SYNTAX score. A large patient level meta-analysis, combining the results of SYNTAX and BEST trials, concluded that CABG offers improved outcomes when compared to DES-PCI in both and non-diabetic and in MVD (2 or 3 vessels involved) with proximal LAD involvement (30).
In conclusion, CABG remains the best revascularization strategy in MVD, conferring reduced mortality and repeat revascularization risk. The absolute risk increases in stroke associated with CABG does not outweigh the benefit in the long-term survival achievable with this technique of revascularization.
The conclusions reached by the currently available studies should be considered carefully when translating the results to the real-life scenarios, which are often inclusive of variegate case mixes with multiple comorbidities. In this context, the importance of the Heart Team is profound.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Makikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-52. [Crossref] [PubMed]
- Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med 2016;375:2223-35. [Crossref] [PubMed]
- Buszman PE, Buszman PP, Banasiewicz-Szkrobka I, et al. Left Main Stenting in Comparison with Surgical Revascularization: 10-Year Outcomes of the (Left Main Coronary Artery Stenting) LE MANS Trial. JACC Cardiovasc Interv 2016;9:318-27. [Crossref] [PubMed]
- Ahn JM, Roh JH, Kim YH, et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease: 5-Year Outcomes of the PRECOMBAT Study. J Am Coll Cardiol. 2015;65:2198-206. [Crossref] [PubMed]
- Morice MC, Serruys PW, Kappetein AP, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation 2014;129:2388-94. [Crossref] [PubMed]
- Putzu A, Gallo M, Martino EA, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention with drug-eluting stents for left main coronary artery disease: A meta-analysis of randomized trials. Int J Cardiol 2017;241:142-8. [Crossref] [PubMed]
- De Rosa S, Polimeni A, Sabatino J, et al. Long-term outcomes of coronary artery bypass grafting versus stent-PCI for unprotected left main disease: a meta-analysis. BMC Cardiovasc Disord 2017;17:240. [Crossref] [PubMed]
- Garg A, Rao SV, Agrawal S, et al. Meta-Analysis of Randomized Controlled Trials of Percutaneous Coronary Intervention with Drug-Eluting Stents Versus Coronary Artery Bypass Grafting in Left Main Coronary Artery Disease. Am J Cardiol 2017;119:1942-8. [Crossref] [PubMed]
- Lee CW, Ahn JM, Cavalcante R, et al. Coronary Artery Bypass Surgery Versus Drug-Eluting Stent Implantation for Left Main or Multivessel Coronary Artery Disease: A Meta-Analysis of Individual Patient Data. JACC Cardiovasc Interv 2016;9:2481-9. [Crossref] [PubMed]
- Nerlekar N, Ha FJ, Verma KP, et al. Percutaneous Coronary Intervention Using Drug-Eluting Stents Versus Coronary Artery Bypass Grafting for Unprotected Left Main Coronary Artery Stenosis: A Meta-Analysis of Randomized Trials. Circ Cardiovasc Interv 2016;9. [PubMed]
- Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol 2015;65:2496-507. [Crossref] [PubMed]
- Park SJ, Ahn JM, Kim YH, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204-12. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Head SJ, Davierwala PM, Serruys PW, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J 2014;35:2821-30. [Crossref] [PubMed]
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010;55:432-40. [Crossref] [PubMed]
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375-84. [Crossref] [PubMed]
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013;61:808-16. [Crossref] [PubMed]
- Benedetto U, Gaudino M, Ng C, et al. Coronary surgery is superior to drug eluting stents in multivessel disease. Systematic review and meta-analysis of contemporary randomized controlled trials. Int J Cardiol 2016;210:19-24. [Crossref] [PubMed]
- Al Ali J, Franck C, Filion KB, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention with first-generation drug-eluting stents: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2014;7:497-506. [Crossref] [PubMed]
- Sipahi I, Akay MH, Dagdelen S, et al. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med 2014;174:223-30. [Crossref] [PubMed]
- Boudriot E, Thiele H, Walther T, et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol 2011;57:538-45. [Crossref] [PubMed]
- Fanari Z, Weiss SA, Zhang W, et al. Comparison of percutaneous coronary intervention with drug eluting stents versus coronary artery bypass grafting in patients with multivessel coronary artery disease: Meta-analysis of six randomized controlled trials. Cardiovasc Revasc Med 2015;16:70-7. Erratum in: J Am Coll Cardiol 2011;57:1792. [Crossref] [PubMed]
- Lim JY, Deo SV, Kim WS, et al. Drug-eluting stents versus coronary artery bypass grafting in diabetic patients with multi-vessel disease: a meta-analysis. Heart Lung Circ 2014;23:717-25. [Crossref] [PubMed]
- Hakeem A, Garg N, Bhatti S, et al. Effectiveness of percutaneous coronary intervention with drug-eluting stents compared with bypass surgery in diabetics with multivessel coronary disease: comprehensive systematic review and meta-analysis of randomized clinical data. J Am Heart Assoc 2013;2. [Crossref] [PubMed]
- D'Ascenzo F, Barbero U, Moretti C, et al. Percutaneous coronary intervention versus coronary artery bypass graft for stable angina: meta-regression of randomized trials. Contemp Clin Trials 2014;38:51-8. [Crossref] [PubMed]
- Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol 2013;62:1421-31. [Crossref] [PubMed]
- Zimarino M, Ricci F, Romanello M, et al. Complete myocardial revascularization confers a larger clinical benefit when performed with state-of-the-art techniques in high-risk patients with multivessel coronary artery disease: A meta-analysis of randomized and observational studies. Catheter Cardiovasc Interv 2016;87:3-12. [Crossref] [PubMed]
- Chang M, Ahn JM, Lee CW, et al. Long-Term Mortality After Coronary Revascularization in Nondiabetic Patients with Multivessel Disease. J Am Coll Cardiol 2016;68:29-36. [Crossref] [PubMed]
- Cavalcante R, Sotomi Y, Zeng Y, et al. Coronary bypass surgery versus stenting in multivessel disease involving the proximal left anterior descending coronary artery. Heart 2017;103:428-33. [Crossref] [PubMed]
- Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939-48. [Crossref] [PubMed]
- Bhatt DL. CABG the clear choice for patients with diabetes and multivessel disease. Lancet 2018;391:913-4. [Crossref] [PubMed]
- Hannan EL, Zhong Y, Berger PB, et al. Association of Coronary Vessel Characteristics With Outcome in Patients With Percutaneous Coronary Interventions With Incomplete Revascularization. JAMA Cardiol 2018;3:123-30. [Crossref] [PubMed]
- Milojevic M, Head SJ, Parasca CA, et al. Causes of Death Following PCI Versus CABG in Complex CAD: 5-Year Follow-Up of SYNTAX. J Am Coll Cardiol 2016;67:42-55. [Crossref] [PubMed]
- Benedetto U, Gaudino M, Di Franco A, et al. Incomplete revascularization and long-term survival after coronary artery bypass surgery. Int J Cardiol 2018;254:59-63. [Crossref] [PubMed]
- Nappi F, Sutherland FW, Al-Attar N, et al. Incomplete Revascularization in PCI and CABG: When Two Plus Two Does Not Make Four. J Am Coll Cardiol 2016;68:877-8. [Crossref] [PubMed]
- Rastan AJ, Walther T, Falk V, et al. Does reasonable incomplete surgical revascularization affect early or long-term survival in patients with multivessel coronary artery disease receiving left internal mammary artery bypass to left anterior descending artery? Circulation 2009;120:S70-7. [Crossref] [PubMed]
- Tarr FI, Sasvari M, Tarr M, et al. Evidence of nitric oxide produced by the internal mammary artery graft in venous drainage of the recipient coronary artery. Ann Thorac Surg 2005;80:1728-31. [Crossref] [PubMed]
- Spadaccio C, Nappi F, Nenna A, et al. Is it time to change how we think about incomplete coronary revascularization? Int J Cardiol 2016;224:295-8. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]





